| Index to this page |
Ozone is a highly active form of oxygen (O3 rather than O2). Ozone is made when a electric spark passes through air, and this accounts for the characteristic odor give off by some electrical motors.
Ozone presents two quite different biological problems: too much at low levels of the atmosphere (the troposphere); too little at high altitudes (the stratosphere).
Ozone is produced by the reaction of sunlight, oxygen, and automobile exhaust (which contains hydrocarbons and nitrogen oxides). Ozone is largely responsible for the discomfort associated with photochemical smog. This form of smog, long familiar to people in the Los Angeles basin, is now common wherever sunlight and stagnant air occur in urban areas (Mexico City is a dramatic example with ozone levels that often exceed 100 ppb and sometimes rise above 350 ppb). High levels of ozone during smog build-up can cause difficulty to people with respiratory ailments like emphysema and asthma. Ozone also damages plants and may be an important factor in the damage that is occurring to forests in Europe and North America.
While we often have too much ozone around us, the concentration of ozone high in the stratosphere (which begins about 7 miles up - where airliners cruise) has declined over the past two decades. Satellite monitoring of the stratosphere, which began in 1978, has revealed a marked decline.
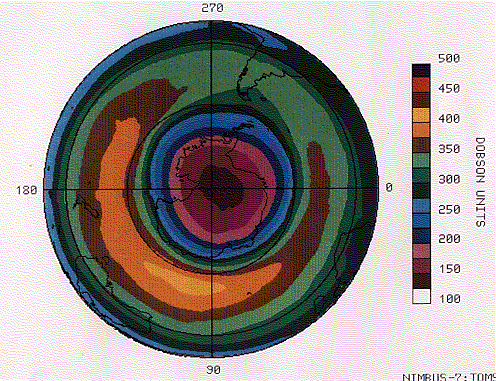
The most serious decline occurs over Antarctica in spring (October) when a precipitous drop in ozone causes an ozone hole.
The figure (courtesy of NASA) shows a map of the ozone hole measured over Antarctica on 5 October 1987 by a device carried on the Nimbus 7 satellite. The tips of South America (upper right quadrant ) and Africa (lower right) are drawn in, as is the outline of Australia and New Zealand (lower left).
The Dobson unit is a measure of the number of molecules of ozone in a vertical column of the atmosphere. You can see that the concentration of ozone decreases in ever-smaller concentric circles with the lowest reading centered over the South Pole.
The spreading of this ozone-depleted air may account for the more gradual and more protracted declines that are being seen at midlatitudes. From 1978-1990, average ozone levels declined 8% over Europe and about 5% over the United States. This is ominous because ozone shields the earth's surface from much of the ultraviolet radiation reaching the earth from the sun. Ultraviolet rays can cause skin cancer, cataracts, and may depress the immune system.
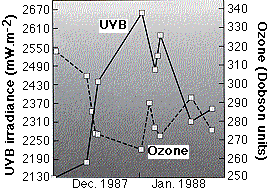 The graph (from C. R. Roy, et. al., in Nature 347:235, 1990) shows measurements of
The graph (from C. R. Roy, et. al., in Nature 347:235, 1990) shows measurements of
Although some of the recent depletion of ozone in the stratosphere was probably due to natural causes (volcanic eruptions, fewer sunspots), some is most likely caused by manmade chlorofluorocarbons (CFCs). These gases escape from such sources as aerosol spray cans, leaky or discarded refrigeration units, and a variety of industrial processes. The U.S., Canada, and the Scandinavian countries stopped using CFCs in aerosol cans over a decade ago, but this and other uses of CFCs have continued to grow worldwide. However, a multi-nation agreement drawn up in 1987 established a schedule for reducing the use of these materials.
And, in fact, monitoring shows that the concentration of CFCs in the stratosphere has been decreasing since the mid-90s.
But the ozone hole itself has not responded in parallel, so CFCs are not the whole story.| Welcome&Next Search |