Culturing Human Embryonic Stem (ES) Cells
In other pages, I describe:
The techniques used in the early steps of each process have now been achieved with human cells.
Human Embryonic Stem (ES) Cells
A research team led by James Thomson of the University of Wisconsin reported in the 6 November 1998 issue of Science that they were able to grow human embryonic stem (ES) cells in culture.
At the time of implantation, the mammalian embryo is a blastocyst.
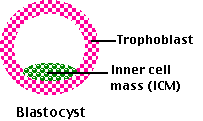 It consists of the
It consists of the
- trophoblast — a hollow sphere of cells that will go on to implant in the uterus and develop into the extraembryonic membranes
- placenta
- umbilical cord
- amnion
- inner cell mass (ICM) that will develop into the baby.
The cells of the inner cell mass are considered pluripotent; that is, each is capable of producing descendants representing all of the hundreds of differentiated cell types in the newborn baby, including
Their process
- Remove the trophoblast cells from a human blastocyst (these were extras not needed for in vitro fertilization (IVF).
- Separate the cells of the inner cell mass and culture them on a plate of "feeder" cells (mouse fibroblasts were used).
- Isolate single cells and grow them as clones.
- Test the clones.
The results
- Each successful clone maintained a normal human karyotype (unlike most cultured human cells — HeLa cells, for example).
- These cells had high levels of the enzyme telomerase, which maintains normal chromosome length and is characteristic of cells with unlimited potential to divide ("immortal").
- When injected into SCID mice, these cells formed teratomas; tumors containing a mix of differentiated human cell types, including cells characteristic of
SCID = severe combined immunodeficiency.
These mice lack a functioning immune system (have neither T cells nor B cells) and so cannot reject foreign tissue. (Some rare inherited diseases of humans are also called SCID. They produce a similar phenotype but involve different molecular defects. [Links]) |
Making ES cells from the differentiated cells of an adult
Researchers at the firm of Advanced Cell Technology (in Worcester, Massachusetts) have reported that they had been able to convert adult human cells into cultured cells that appear to have the properties of embryonic stem cells; that is, pluripotent and, perhaps, "immortal".
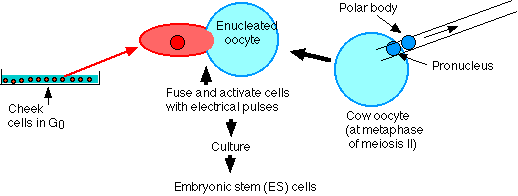
Their process is quite like that used to produce Dolly.
- Cells were removed from an adult human (Jose Cibelli, one of the researchers). Both his cheek cells and his white blood cells were tried.
- Cow oocytes were enucleated.
- Human cell and enucleated egg were fused using a pulse of electricity.
- The resulting cell was grown in culture.
The Goals of These Achievements
Both these procedures have been used with other animals (mice and sheep). In both those cases, the products were implanted in the uterus of the host animal and grew into a complete animal (cloned mice and Dolly, respectively). Do these workers plan to do the same with their human cells? They assure us that they do not.
So what are their goals?
Human embryonic stem cells have the potential to
- teach us about the process of human embryonic development, its genetic control, etc.
- If the proper signals can be discovered, it may be possible to cause these cells to differentiate along a particular pathway, e.g., to form insulin-secreting beta cells of the islets of Langerhans.
- Such cells might be able to replace lost or non-functioning cells in a human patient (e.g., with insulin-dependent diabetes mellitus — IDDM).
- Such cells might be transformed with the DNA needed to express a gene missing in the transplant recipient.
- Such cells might be engineered to reduce the risk of transplant rejection.
Gametes from human ES cells?
Working with mice, several laboratories report that they have been able to coax ES cells to differentiate into cells with some of the properties of gametes, including
- becoming haploid;
- erasing the methylation of imprinted genes;
- being able to mimic fertilization and support development of a blastocyst.
If this can be achieved with humans, it could enable the infertile member of a couple to generate a gamete from one of his or her somatic cells.
Procedure:
- Remove a somatic cell (e.g., skin) nucleus from infertile donor.
- Inject it into an enucleated oocyte.
- Culture until a blastocyst is formed.
- Harvest embryonic stem (ES) cells from the inner cell mass.
- Culture them under the conditions that will cause them to differentiate into gametes.
- Fertilize these with gametes from the other parent by IVF
- Grow them in culture until a few are ready to implant in a uterus.
- Hope for the best.
If, as seems to be the case with mice, ES cells — whether derived from a male or a female — can be cultured to differentiate into either sperm or oocytes, then there even exists the possibility of two men being able to be the parents of a child.
29 September 2004
 It consists of the
It consists of the
