While some success has been achieved with laboratory animals, not much has yet been achieved with humans.
One exception: culturing human epithelial stem cells and using their differentiated progeny to replace a damaged cornea. This works best when the stem cells are from the patient (e.g. from the other eye). Corneal cells from another person (an allograft) are always at risk of rejection by the recipient's immune system.
One way to avoid the problem of rejection is to use stem cells that are genetically identical to the host.
This is already possible in the rare situations when the patient has healthy stem cells in an undamaged part of the body (like the stem cells being used to replace damaged corneas).
But even where no "autologous" stems cells are available, there may be a solution: using - Imprinted Genes.
Sperm and eggs each contain certain genes that carry an "imprint" identifying them later in the fertilized egg as being derived from the father or mother respectively.
Creating an egg with a nucleus taken from an adult cell may not allow a proper pattern of imprinting to be established.
When the diploid adult nucleus is inserted into the enucleated egg (at least those of sheep and mice), the new nucleus becomes "reprogrammed". What reprogramming actually means still must be learned, but perhaps it involves the proper methylation and demethylation of imprinted genes. For example, the inactive X chromosome in adult female cells must be reactivated in the egg, and this actually seems to happen.
- Aneuploidy.
In primates (in contrast to sheep, cattle, and mice), the process of removing the resident nucleus causes molecules associated with the centrosome to be lost as well. Although injecting a donor nucleus allows mitosis to begin, spindle formation may be disrupted, and the resulting cells fail to get the correct complement of chromosomes (aneuploidy).
- Somatic Mutations.
This procedure also raises the spectre of amplifying the effect(s) of somatic mutations. [Link to discussion]
In other words, mutations that might be well-tolerated in a single somatic cell of the adult (used to provide the nucleus) might well turn out to be quite harmful when they become replicated in a clone of cells injected later into the patient.
- Political Controversy.
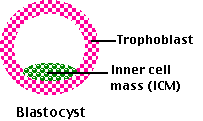
The goal of this procedure (which is often called "therapeutic cloning" even though no new individual is produced) is to culture a blastocyst that can serve as a source of ES cells.
But that same blastocyst could theoretically be implanted in a human uterus and develop into a baby that was genetically identical to the donor of the nucleus. In this way, a human would be cloned.
And in fact, Dolly and other animals are now routinely cloned this way. Link to a description.
The spectre of this is so abhorrent to many that they would like to see the procedure banned despite its promise for helping humans.
In fact, many are so strongly opposed to using human blastocysts — even when produced by nuclear transfer — that they would like to limit stem cell research to adult stem cells (even though these are only multipotent).
Two possible solutions (both so far demonstrated only in mice):
- ES cells can be derived from a single cell removed from an 8-cell morula. We know that, in humans, removing a single cell from the morula does not destroy it — the remaing cells can develop into a blastocyst, implant, and develop into a healthy baby. [link to evidence].
- In altered nuclear transfer (ANT) — a modified version of SCNT (somatic-cell nuclear transfer) — a gene necessary for later implantation (Cdx2 — endocoding a homeobox transcription factor) is turned off (by RNA interference) in the donor nucleus before the nucleus is inserted into the egg. The blastocyst that develops
- has a defective trophoblast that cannot implant in a uterus;
- but the cells of the inner cell mass are still capable of developing into cultures of ES cells. (The gene encoding the interfering RNA can then be removed using the Cre/loxP technique.)
If these procedures work in humans, neither would involve the destruction of a potential human life.