This page examines the detection of heat, cold, and pain.
Why pain? Because at least some of the receptors of heat and cold — when the stimulus exceeds a certain threshold — transmit signals that the brain interprets as pain.
Few, if any, of the detectors of heat, cold, and pain are specialized transducers (in the way that, for example, the Pacinian corpuscle is). Rather they seem to be the endings of sensory neurons whose activation is determined by transmembrane proteins embedded in their plasma membrane. A single neuron may have several types of these receptors and thus be able to respond to several types of stimuli. Like all sensory spinal neurons, their axons travel to a dorsal root ganglion of the spinal cord, where their cell bodies reside, and then on in to the gray matter of the spinal cord.
There are several types of heat receptors in the skin. They are all transmembrane proteins in the plasma membrane that open to let in both calcium ions and sodium ions (the latter the source of the action potential). Between them, they cover a range of temperatures.
- TRPV4
Warm (>25°C)
- TRPV3
Warmer (>31°C)
- TRPV1 (also known as VR1)
Hot (>43°C). Also activated by capsaicin, the active ingredient of hot chili peppers, by camphor, and by acids (protons).
- TRPV2 (also called VRL-1)
Painfully hot (>52°C)
Knockout mice lacking the TRPV1 receptor not only do not avoid water with capsaicin in it but have a diminished response to heat.
Birds also have TRPV1 receptors. Theirs also respond to heat (and acids), but do not respond to capsaicin. This must explain why birds happily eat hot chili peppers (and so disperse their seeds).
Probably two types of receptors.
- One, designated TRPM8 (or CMR1), is a channel that admits Ca2+ and Na+ in response to moderate cold (<28°C) or menthol (the ingredient that gives mint its "cool" touch and taste).
- A second, designated TRPA1 (or ANKTM1), responds to lower temperatures (<18°C) and elicit signals that the brain interprets at pain. (TRPA1 is also found in the hair cells of the inner ear that respond to sound and changes in position.)
When sensory nerve fibers are exposed to extremes, they signal pain. Pain receptors are also called nociceptors.
]
Two types of sensory nerve fibers transmit signals that the brain interprets as pain.
- Aδ ("A-delta") fibers
- These are thinly-myelinated.
- Their activation is rapid and associated with acute pain. This is "good pain" because it warns you to do something to take care of the problems, e.g., a hot saucepan.
- C fibers
- These are unmyelinated and thus conduct impulses slowly.
- Their activation is slower and associated with diffuse, dull, chronic pain. This is "bad pain" because it cannot be alleviated simply by removing the stimulus. It is pain generated by such things as damaged tissue and cancer.
Both Aδ and C fibers are part of the sensory-somatic branch of the peripheral nervous system. Their axons pass into the dorsal root ganglion, where their cell body is located, and then on in to the gray matter of the spinal cord where they synapse with interneurons.
Several different neurotransmitters have been implicated in pain pathways. Three of them:
- glutamate. This seems to be the dominant neurotransmitter when the threshold to pain is first crossed. It is associated with acute ("good") pain.
- substance P. This peptide (containing 11 amino acids) is released by C fibers. It is associated with intense, persistent, chronic — thus "bad" — pain.
- glycine. It suppresses the transmission of pain signals in the dorsal root ganglion. Prostaglandins potentiate the pain of inflammation by blocking its action.
The brain can also register pain from stimuli originating in sensory neurons of the autonomic nervous system. This so-called visceral pain is not felt in a discrete location as pain signals transmitted by the sensory-somatic system are.
The weapons presently available to reduce pain are many in number but few in types. They are
- Non-steroidal anti-inflammatory drugs (NSAIDs)
- Opioids (also called opiates)
Inflammation is caused by tissue damage and, among other things, causes pain. Damaged tissue releases prostaglandins and these are potent triggers of pain.
Prostaglandins are 20-carbon organic acids synthesized from unsaturated fatty acids.
There are at least three key enzymes that synthesize prostaglandins:
- Cyclooxygenase 1 (Cox-1)
- Cyclooxygenase 2 (Cox-2)
- Cyclooxygenase 3 (Cox-3)
Most NSAIDs block the action of all three cyclooxygenases.
They include:
- aspirin
- ibuprofen (Advil®, Motrin®)
- naproxen (Aleve®)
- and many others
Two NSAIDs
- celecoxib (Celebrex®)
- rofecoxib (Vioxx®)
were introduced in 1999 that selectively inhibit Cox-2 while leaving Cox-1 untouched. It was hoped that these would provide pain relief without the gastrointestinal side effects associated with the broad spectrum NSAIDs. However, the manufacturer of Vioxx® removed it from the market on 30 September 2004 because it increases the risk of heart attacks and strokes.
This is also a nonsteroidal anti-inflammatory drug but its mode of action is different from the others. It selectively inhibits Cox-3 and provides pain relief without irritating the stomach. It is particularly useful for
- people allergic to aspirin and its relatives
- avoiding the risk of Reye's syndrome that has been associated with giving aspirin to children with viral infections.
Opioids are extremely effective pain killers but are also addictive so their use is surrounded with controversy and regulation.
Some examples:
- morphine
- codeine
- methadone
- demerol
- darvon
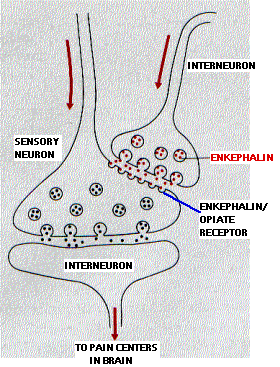
Opioids bind to receptors on interneurons in the pain pathways in the central nervous system. The natural ligands for these receptors are two enkephalins — each a pentapeptide (5 amino acids):
- Met-enkephalin (Tyr-Gly-Gly-Phe-Met)
- Leu-enkephalin (Tyr-Gly-Gly-Phe-Leu)
The two enkephalins are released at synapses on neurons involved in transmitting pain signals back to the brain. Instead of synapsing with a dendrite or cell body, the enkephalin synapse occurs close to the terminal of a pain-signaling neuron. The enkephalins hyperpolarize the postsynaptic membrane thus inhibiting it from transmitting these pain signals.
The drawing shows how this mechanism might work. The activation of enkephalin synapses suppresses the release of the neurotransmitter (substance P) used by the sensory neurons involved in the perception of chronic and/or intense pain.
The ability to perceive pain is vital. However, faced with massive, chronic, intractable pain, it makes sense to have a system that decreases its own sensitivity . Enkephalin synapses provide this intrinsic pain suppressing system.
Morphine and the other opioids bind these same receptors. This makes them excellent pain killers.
However, they are also highly addictive.
- By binding to enkephalin receptors, they enhance the pain-killing effects of the enkephalins.
- A homeostatic reduction in the sensitivity of these synapses compensates for continued exposure to opioids.
- This produces tolerance, the need for higher doses to achieve the prior effect.
- If use of the drug ceases, the now relatively insensitive synapses respond less well to the soothing effects of the enkephalins, and the painful symptoms of withdrawal are produced.
Research is progressing on coupling substance P to a cytotoxin.
The plan:
- Inject the conjugate into the cerebrospinal fluid so that it can
- bind to substance P receptors in the spinal cord and
- be taken in by endocytosis.
- Once inside, the toxin portion of the conjugate kills the cell
- thus interrupting the pathway that mediates chronic, intractable pain while leaving untouched the "good pain" pathways.
23 October 2005
