BIOL 1400 -- Lecture Outline 31
Click here to view the slide show
for this lecture
"Against stupidity the gods themselves fight in vain." -- Johann von Schiller
I. What is life made of?
- The German chemist Friederich Wöhler made the compound urea
in his laboratory in 1828.
- Urea is a waste product found in mammal urine. . . but Wöhler made
urea starting with chemicals that had never been part of a living thing.
- Who cares?
- Many scientists at the time thought that life was caused by some "vital force"
which made living matter fundamentally different from nonliving matter.
- Wöhler's experiment -- and countless more that followed -- showed
that living things aren't different from nonliving things at the chemical level.
The same atoms are found in both, and both follow the same basic laws of chemistry.
- Living things are chemically very complex, much more complex than typical nonliving
things -- but this is a difference of degree, not of kind.
- By 1800, chemists had discovered many ways to break down substances found in nature, and to create new substances.
- Some substances could not be broken down into anything else. They might combine
to make new materials, but they could not be broken down into anything simpler.
- These are the chemical elements.
- Common examples include: gold, silver, copper, aluminum, iron, sulfur,
carbon, oxygen, helium. . .
- There are now known to be 88 elements found in nature.
- Four of them -- carbon, nitrogen, oxygen, and hydrogen (CHON)-- make up over 95%
of your body. Two others -- sulfur and phosphorus (SP) are also very important.
- Others (iron, calcium, potassium, zinc, etc.) play subordinate but still important roles.
- There is a standard system for abbreviating elements. Usually, but not always,
we use the first letter or first two letters of the element's name. Examples: carbon
= C; hydrogen = H; oxygen = O; nitrogen = N; helium= He; neon = Ne; gold = Au (from its
Latin name aurum). . . and so on. Check out
this
table of the elements if you want more information. . .
- Chemists like John Dalton (1803) noticed that elements combined with other
elements to make compounds -- new substances with very different
properties -- but only in fixed ratios of small whole numbers.
Different compounds are made of different ratios of elements.
- Simple example: If you send an electric current through some water, you
will break it up into hydrogen and oxygen. . .
- . . . and you will always get exactly twice as much hydrogen as oxygen, no
more and no less.
- What this suggests is that elements are made up of atoms --
tiny particles which can attach to each other, but only in specific and definite ways.
(An attachment between two atoms is a chemical bond, and we'll cover those
shortly.)
- Two hydrogen atoms can stick to one oxygen atom, and that gives you
a molecule of water. (We write this as the familiar H2O.)
- You can also stick two hydrogen atoms to two oxygen atoms, but that
does not give you water. It gives a compound known as hydrogen peroxide,
H2O2 (used to disinfect cuts and wounds, and to bleach hair,
among other things).
- Conclusion: A compound is determined by the numbers, arrangements, and types
of atoms that make it up. Change that, and you change the compound.
II. OK, so we got atoms. . . is that all there is?
- Around 1900, radioactivity was discovered.
- Some atoms aren't stable -- they break up, and throw out "pieces" of themselves.
- Other atoms are usually stable but can be forced to break up.
- The "pieces" -- radiation and subatomic particles -- "thrown out" by atoms
breaking up can be detected as radioactivity.
- And the upshot is that atoms aren't indivisible -- they can be broken into even
smaller pieces.
- By 1935, scientists had isolated and named the "pieces", a.k.a.
subatomic particles.
- Three kinds of stable subatomic particles make up atoms:
- Proton -- a particle with a positive electric charge
- Electron -- a particle with a negative electric charge
- Neutron -- a particle with no electric charge
- The number of protons (the atomic number normally equals the
number of electrons. If it doesn't--if an atom is missing an electron, or it has
an extra -- the atom has an electric charge on it, and is called an ion.
- OK, but how are these particles arranged in an atom?
- Old "plum pudding" model had all three types of particles clumped together,
forming a sort of lump.
- Famous experiment by Ernest Rutherford disproved this model. Rutherford
showed that an atom is mostly empty space. (!?!)
- The protons and neutrons are clustered together in the center, or
atomic nucleus,
- . . . . while the electrons are whizzing around the nucleus in orbits.
- If you could somehow enlarge an atom to the size of the Louisiana
Superdome in New Orleans, the electrons would be whizzing around the
roof, and the nucleus would be the size of a pea on the 50-yard-line. Most of
an atom is empty space.
- But electrons don't just orbit anywhere. They can only exist at certain
energy levels, called orbitals. (Analogy: You can only live on the first,
second, third, etc. floor of a building; there is no such thing as a one-half
floor, 2.35th floor, 3.14159th floor.)
- "Solar system" model of an atom looks like this. Notice that the electrons
are in one of two orbitals:
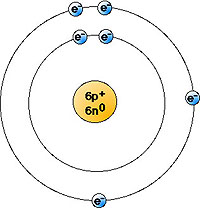
This represents an atom of carbon-12: six protons, six neutrons, six electrons
- From the point of view of a nuclear physicist, this is not really an accurate
model. Atoms don't "really look" like this (the nucleus is WAYYYYY
too big, and the orbitals of electrons are NOT neat circles). However, it
clearly shows those features of an atom that we will need to
apply to biology.
- Chemical Bonding
- Each orbital can only accommodate a set number of electrons.
- However, atoms can share electrons between outer orbitals, so that both fill the
available spaces in their outer orbitals.
- A bond between atoms that's caused by sharing electrons is a covalent
bond
- Example: This is a molecule of methane, the main ingredient in
natural gas (formula CH4):

- Covalent bonds can be drawn as short lines:
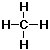 . When there's no
confusion possible, the lines can be left out: CH4.
. When there's no
confusion possible, the lines can be left out: CH4.
- c. Your text also uses "bubble diagrams" to represent molecules.
Methane would look like this:

- Most of the molecules we will be interested in are held together by covalent
bonds, and most of the chemical reactions we will study involve breaking and
reforming covalent bonds.
III. Chemical Energy
- Energy takes a lot of forms: heat, light, motion, electricity. . .
But all can be classified into two types: kinetic energy and potential
energy.
- The First Law of Thermodynamics states that energy can neither be
created or destroyed, but can be changed from one form to another.
- Example: A nuclear power plant turns nuclear energy (the energy possessed by
subatomic particles) into heat energy (which heats water and turns it into steam).
The steam drives a turbine (turning heat into motion) which runs an electrical
generator (turning motion into electricity, the energy carried by a flow of electrons
through a wire). Plug a light bulb into the wire, and the electrical energy is turned
into light and heat. Plug a battery and battery charger into the wire, and the
kinetic electrical energy is stored as potential energy.
- Energy tends to move from areas of high concentration to areas of low
concentration. Example: Light a fire and you slowly warm the room, as the heat
energy (actually, the motion of the molecules of air) diffuses outward.
- Potential energy can be stored in chemical bonds. That energy is released when
the bonds are broken. On the other hand, energy is also required to form chemical
bonds.
- Energetics of Chemical Reactions
- If a chemical reaction releases more energy than it consumes, the reaction is
exothermic (another word you might see for nearly the same thing is
exergonic).
- Example: When you burn gasoline, what you're actually doing is breaking
bonds between the carbon and hydrogen atoms in the gasoline molecules, and
forming new bonds between these atoms and atoms of oxygen in the atmosphere.
Assuming it's a clean burn, the reaction looks something like this:
2 C8H18 + 25 O2 ->
16 CO2 + 18 H2O + heat and light energy
- On the other hand, a reaction that consumes more energy than it releases is
endergonic.
- But an exergonic reaction may still need an input of energy to get started --
a "kickstart," if you will. This is the activation energy.
Example: You have
to light a match, or fire an electric spark, to start gasoline burning.
- Once the
reaction is started, it will continue until the gasoline (or oxygen!) is used up,
releasing energy all the while -- but gasoline won't normally spontaneously
combust unless activation energy starts the reaction going.
- However, some substances will lower the activation energy of a reaction,
making it much easier to start. These are catalysts.
- Try this at home: Try setting a sugar cube on fire with a match. It probably
won't work. Next, rub the sugar cube with a little paper ash, and try lighting it
again. It should work this time. The ash is acting as a catalyst.
- Example 2: The catalytic converter of a modern car engine contains platinum.
Platinum acts as a catalyst to speed up the conversion of harmful carbon monoxide
to relatively harmless carbon dioxide.
Go to Previous Notes |
Return to Lecture Schedule |
Return to Syllabus |
Contact the Prof |
Go to Next Notes


 . When there's no
confusion possible, the lines can be left out: CH4.
. When there's no
confusion possible, the lines can be left out: CH4.
