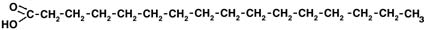BIOL 1400 -- Lecture Outline 32
"What luck for rulers, that men do not think ." -- Adolf Hitler
I. The Chemistry of Life: Simple Molecules
- Life processes require atoms of various elements (sodium, iron, potassium,
chlorine, etc.) for various purposes -- most of which we'll look at later on.
- By far the single most important molecule for life is WATER.
- Just to show how important water is: Your body is about 60% water by
weight, and you will take in about 16,000 gallons of water over your lifetime.
- To explain why water's so important, we must cover a second type of
biologically important bonding: hydrogen bonds
- Atoms bound by a covalent bond don't always "share and share alike" -- they
do not always share electrons evenly.
- If this is the case, electrons will spend more time orbiting one atom than
the other.
- The atom with the greater affinity for electrons will thus carry a slight
negative charge. And the atom with less affinity for its electrons will bear
a slight positive charge.
- A molecule that has these slight charges is said to be a polar molecule.
- Water is a good example of this phenomenon. Oxygen has a high electron affinity, and
tends to pull the shared electrons towards itself.
- Because of a bend in the water molecule, one end of each molecule -- where
the oxygen atom is -- is more negative than the other.
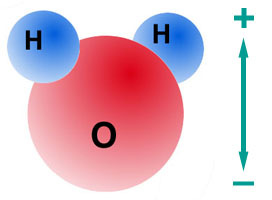
Space-filling model of a water molecule. Note how the bend makes it
possible for one end of the molecule to be more negative than the other.
- Thus, weak bonds form between positive and negative parts of water molecules.
These are hydrogen bonds.
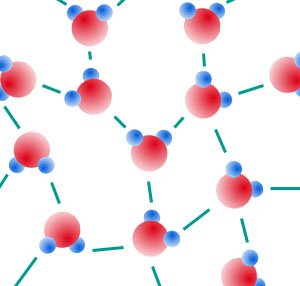
Model of liquid water. If this were solid water (ice), the molecules would
be arranged in a much more regular, crystalline fashion.
- Hydrogen bonds can form between other things, too -- for instance, they lock two
DNA strands together into the "double helix" shape.
- It's this polarity, and its ability to form hydrogen bonds, that are responsible
for some of the unusual properties of water:
- It's a good solvent (i.e. it dissolves many substances)
- It makes nonpolar molecules clump up (e.g. blobs of oil in your salad
dressing) because nonpolar molecules have no charge and are not attracted to
water molecules. Such nonpolar molecules are said to be hydrophobic.
- It has high cohesion (tendency to stick together)

This bug, a water strider, is able to walk on the surface of water
because of its cohesion.
- It has a high specific heat (i.e. it takes a lot of energy to raise
its temperature), a high heat of vaporization (i.e. it takes a lot of energy to
evaporate it) and a high heat of fusion (i.e. it must lose a lot of energy
to freeze it)
- A molecule that has a charge on it -- either an ion, or a polar molecule --
will be attracted to water molecules. Such a molecule is hydrophilic.
- A nonpolar molecule -- e.g. gasoline, oils -- will not be attracted to water
molecules. Those molecules are hydrophobic.
- Molecules with both hydrophobic and hydrophilic parts are known as
amphipathic. EXAMPLE: Soap.
II. Complex Molecules
- There are four basic classes of complex molecule that are important in
living systems. Two of these you know something about already -- proteins and
nucleic acids. Here's a swift look at the other two. . .
- Carbohydrates
- As the name suggests, carbohydrates are composed of carbon, hydrogen, and oxygen
(and usually only these elements)
- Carbohydrate monomers are simple sugars. Usually these are drawn as
ring-shaped molecules, although the rings can unlink (Fig. 3-2). EXAMPLE: glucose
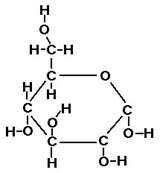
Glucose
- More complex sugars are dimers (two monomers joined) and trimers
(three monomers joined). EXAMPLE: sucrose (table sugar), lactose (milk sugar)
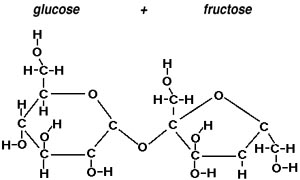
Sucrose (a dimer of glucose + fructose)
- Longer polymers are known as starches (found in plants) or glycogen
(found in animals, including you).
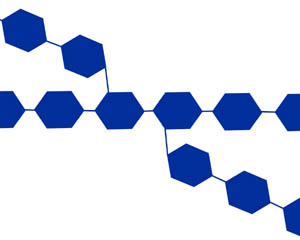
Model of glycogen, a branching polymer of simple sugar units (represented
by hexagons)
- A really long, branched, fibrous carbohydrate polymer is cellulose (what wood and
paper are mostly made of; also "dietary fiber". )
- A final noteworthy polysaccharide is chitin, found in fungi, and also in the outer
skeletons of insects -- it's what makes cockroaches go crunch when you stomp them.
- Functions of carbohydrates: Energy sources, energy storage, and structural support
- Lipids -- three classes of these:
- Oils, fats, and waxes
- Start with a long carbon-hydrogen chain, with a -COOH group at the end.
This is a fatty acid. Here's one:
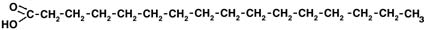
A fatty acid
(By the way, we can save some space by drawing the molecule this way:
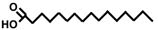
The same fatty acid.
Each corner of the zigzag represents one carbon atom and a full set of hydrogen atoms.)
- You can stick three fatty acids onto one molecule of the three-carbon
alcohol, glycerol. This produces a fat
(solid at room temperature) or an oil (liquid at room temperature). Both are
known as triglycerides.
- If you stick fatty acids onto a larger type of alcohol molecule, you get a
wax. (EXAMPLES: Beeswax, ear wax, waxy coatings on some plant leaves)
- Phospholipids
- These are similar to oils and fats, except there are only two fatty acids attached
to the glycerol molecule.
- The third carbon of the glycerol is attached to a polar group, containing phosphorus
and nitrogen.
The result is that a phospholipid has two fatty acids, which form
nonpolar, hydrophobic "tails", and a polar, hydrophilic "head" which contains
oxygen and phosphorus.
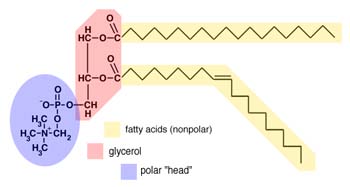
A typical phospholipid
- When you put phospholipids in water, the molecules will arrange themselves
so that the "heads" face the water, and the "tails" are as far away from water
molecules as possible.
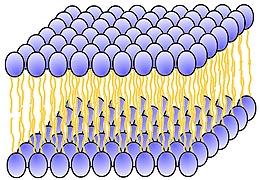
A phospholipid bilayer membrane
- If there are enough phospholipids, a two-layered structure can form, with
all the "heads" facing the water, and all the "tails" away from the water.
- What's more, various proteins and glycoproteins (protein-carbohydrate
combinations) are embedded in this bilayer membrane. More later on what they're
doing there!
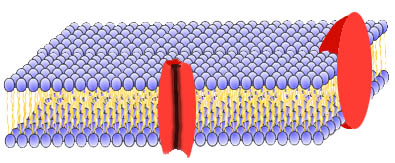
A phospholipid bilayer membrane with embedded proteins (shown
in red)
- Sterols and steroids
- These are not like the other two classes.
- They are based on multiple carbon ring structures. EXAMPLE: testosterone
(male sex hormone, also used as an illicit muscle-building supplement).
- Functions: Some are cell membrane components, such as the infamous
cholesterol -- but most are chemical signaling molecules known as
hormones (although not every hormone is a steroid).
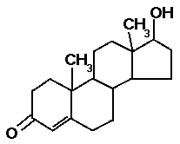
Testosterone
Notice how this molecule is made of four carbon rings of five or six carbons each.
(The actual carbon atoms, and the hydrogens they are bound to, are omitted for
clarity -- but at every corner of each ring, there is a carbon atom.)
Go to Previous Notes |
Return to Lecture Schedule |
Return to Syllabus |
Contact the Prof |
Go to Next Notes