BIOL 1400 -- Lecture Outline 33
"A person has only so much knowledge as he puts to work." -- St. Francis of Assisi
I. Quick review of proteins
- The monomers that make up proteins are called amino acids.
- Each amino acid has one amino group (NH2-)
and one carboxyl group (-COOH), both attached to a central carbon atom.
- Each amino acid also has another group of atoms, called the R-group,
attached to the central carbon.
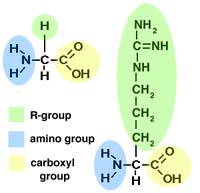
Two sample amino acids (glycine on the left, arginine on the right)
- An amino group on one amino acid may attach to the carboxyl group on another.
This is called a peptide bond.
- Review of protein shape. . .
- The sequence of amino acids is a protein's primary structure. The
picture below, a so-called stick model of a molecule, shows peptide
bonds in blue and carbon atoms in green.
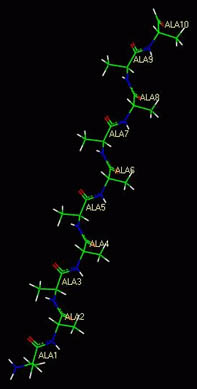
A polymer of animo acids
- Hydrogen bonds (remember those?) between oxygen and nitrogen
atoms in the carboxyl and amino groups can cause the amino acid chain to fold or curl
up into its secondary structure. (Examples are the alpha-helix and
the beta-sheet.)
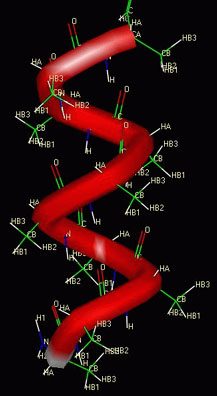
A polymer of animo acids coiled up in an alpha-helix

A polymer of animo acids folded up in a beta-sheet
- Polar R-groups may attract each other, nonpolar R-groups may be repelled
from polar R-groups. Sometimes R-groups form covalent bonds with each other,
which puts a kink or loop in the polypeptide. This causes the
protein to fold up even further. This is its tertiary structure.
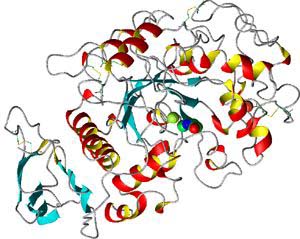
A protein's tertiary structure. You can see several alpha-helix regions
(red and yellow) and beta-sheet regions (blue)
- And finally, different polypeptides may attach to each other, to form a large
molecule; this is the quaternary structure.
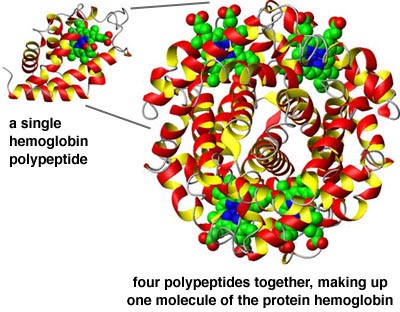
The protein hemoglobin is made up of several polypeptides
- If you alter a protein's environment, you can cause it to change structure,
in particular its tertiary structure. This is called denaturing the protein.
- Finally, many proteins bond with additional "helper" molecules that aren't
proteins but that "assist" the protein in its function. These are called cofactors.
- These may include metal atoms. EXAMPLE: Hemoglobin cannot function without
iron; key proteins involved in manufacturing DNA cannot function without zinc.
- Many vitamins, such as vitamin B6 and vitamin C, are organic
cofactors, a.k.a. coenzymes.
- As you shoudl know by now, the POINT is this: The shape of a protein molecule
enables it to function.
II. Enzymes and enzyme activity
- Enzymes are catalysts. . .
- Catalysts cause certain chemical reactions to happen
by lowering their activation energy. (See Notes 31 if you
need a refresher on what activation energy is. . . )
- Usually, a given enzyme only catalyzes one chemical reaction. This activity
is dependent on the shape of the enzyme molecule.
- The molecule or molecules with which an enzyme interacts are called the
substrate.
- Usually, the enzyme has to fit closely to the substrate molecules;
this is the "lock and key" model. The substrate molecules fit into the
active site of the enzyme.
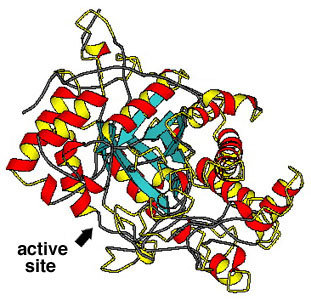
The enzyme beta-amylase
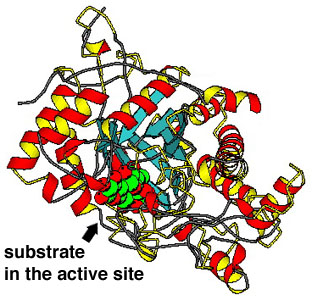
The enzyme beta-amylase with a sugar molecule (in green) bound to its
active site
- An enzyme might bring together two molecules so that they can form a bond
and join together. . .
- . . . or it might "stretch" one molecule so that it breaks into two.
- In either case, since the protein is a catalyst, it is not used up by the reaction.
- An enzyme's activity can be regulated in several ways:
- Competitive inhibition -- two or more molecules will bind to
an enzyme's binding site (some poisons work this way)
- Allosteric regulation -- another molecule binds to the enzyme,
not at the active site, but causing the enzyme molecule to change shape
- A cell may use either of these to control the chemical reactions going on
inside it. A common pattern is feedback inhibition, in which an enzyme
that helps form a certain product is inhibited by that product.
III. How do cells take in the molecules they need?
- Remember that a cell is bounded by a plasma membrane of phospholipids.
There are many kinds of membrane proteins embedded
in the lipid-bilayer membrane.
- Transport proteins -- regulate the movement of molecules across
a membrane
- Receptor proteins -- allow a cell to react to hormones, vitamins,
chemical triggers, etc.
- Recognition proteins -- the cell's "dogtags." Allows your immune system
to recognize and attack foreign cells
- A plasma membrane is differentially permeable, which is a
fancy-pants way of saying that it lets some things through and keeps other
things out.
- There are several ways molecules can enter or leave a cell that don't
require the cell to use energy.
- Some molecules are small enough to cross a bilayer directly -- e.g. water,
ethyl alcohol, oxygen, carbon dioxide, etc. This is simple diffusion.
- Simple diffusion of water across a membrane has a special name:
osmosis.
- Other molecules cannot normally pass through a membrane, but there are
proteins that act as channels for those molecules. This is particularly true for
ions of sodium (Na+), potassium (K+), chlorine (Cl-),
and others. This is facilitated diffusion.
- In all of these cases, net movement of the molecule across the membrane
will only happen until the concentration of the molecule is the same on both
sides.
- Cells may have to expend energy to get other things they need.
- Some membrane proteins act as "pumps" to force molecules into a cell against
a gradient. This is active transport.
- A cell may also engulf large molecules, fluid droplets, or large particles.
This is endocytosis. (It's also called phagocytosis if the thing
the cell's engulfing is relatively large, or pinocytosis if it's tiny. . . but
this is just jargon; don't worry about these terms.)
Go to Previous Notes |
Return to Lecture Schedule |
Return to Syllabus |
Contact the Prof |
Go to Next Notes







