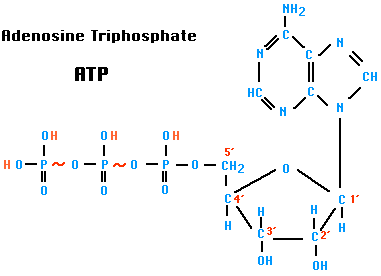
When the third phosphate group of ATP is removed by hydrolysis, a substantial amount of free energy is released. The exact amount depends on the conditions, but we shall use a value of 7.3 kcal per mole.
ATP + H2O → ADP + PiADP is adenosine diphosphate. Pi is inorganic phosphate. [structure]
For this reason, this bond is known as a "high-energy" bond and is depicted in the figure by a wavy red line. (The bond between the first and second phosphates is also "high-energy".) (But please note that the term is not being used in the same sense as the term "bond energy". In fact, these bonds are actually weak bonds with low bond energies.)
| Discussion of bond energy. |
| Welcome&Next Search |