Two theories — with many subtheories — attempt to account for the origin of life on our earth.
- Spontaneous generation somewhere on the early earth
- Panspermia: brought to earth from space.
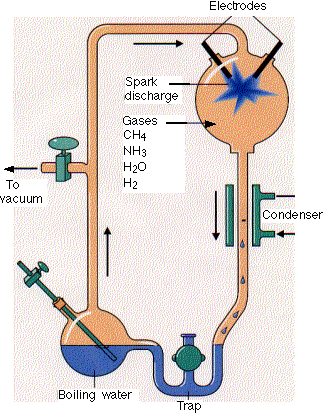 Stanley Miller, a graduate student in biochemistry, built the apparatus shown here. He filled it with
Stanley Miller, a graduate student in biochemistry, built the apparatus shown here. He filled it with - water (H2O
- methane (CH4)
- ammonia (NH3) and
- hydrogen (H2)
- but no oxygen
He hypothesized that this mixture resembled the atmosphere of the early earth. (Some are not so sure.) The mixture was kept circulating by continuously boiling and then condensing the water.
The gases passed through a chamber containing two electrodes with a spark passing between them.
At the end of a week, Miller used paper chromatography to show that the flask now contained several amino acids as well as some other organic molecules.
In the years since Miller's work, many variants of his procedure have been tried. Virtually all the small molecules that are associated with life have been formed:
- 17 of the 20 amino acids used in protein synthesis, and
- all the purines and pyrimidines used in nucleic acid synthesis.
- But abiotic synthesis of ribose — and thus of nucleosides — has been much more difficult.
One difficulty with the primeval soup theory is how polymers — the basis of life itself — could be assembled.
- In solution, hydrolysis of a growing polymer would soon limit the size it could reach.
- Abiotic synthesis produces a mixture of L and D enantiomers. Each inhibits the polymerization of the other. (So, for example, the presence of D amino acids inhibits the polymerization of L amino acids (the ones that make up proteins here on earth).
This has led to a theory that early polymers were assembled on solid, mineral surfaces that protected them from degradation, and in the laboratory polynucleotides and polypeptides containing about ~50 units have been synthesized on mineral (e.g., clay) surfaces.
All metabolism depends on enzymes and, until recently, every enzyme has turned out to be a protein.
But proteins are synthesized from information encoded in DNA and translated into mRNA.
So here is a chicken-and-egg dilemma. The synthesis of DNA and RNA requires proteins. So - proteins cannot be made without nucleic acids and
- nucleic acids cannot be made without proteins.
The discovery that certain RNA molecules have enzymatic activity provides a possible solution.
These RNA molecules — called ribozymes — incorporate both the features required of life:
- storage of information
- the ability to act as catalysts
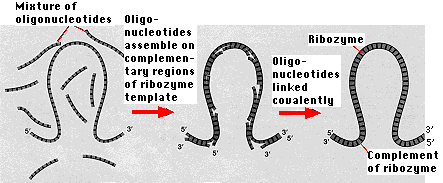
While no ribozyme in nature has yet been found that can replicate itself, ribozymes have been synthesized in the laboratory that can catalyze the assembly of short oligonucleotides into exact complements of themselves. The ribozyme serves as both
- the template on which short lengths of RNA ("oligonucleotides" are assembled following the rules of base pairing and
- the catalyst for covalently linking these oligonucleotides.
(The figure is based on the work of Green and Szostak, Science 258:1910, 1992.)
In principal, the minimal functions of life might have begun with RNA and only later did
- proteins take over the catalytic machinery of metabolism and
- DNA take over as the repository of the genetic code.
Several other bits of evidence support this notion of an original "RNA world":
- Many of the cofactors that play so many roles in life are based on ribose; for example:
- In the cell, all deoxyribonucleotides are synthesized from ribonucleotide precursors.
- Many bacteria control the transcription and/or translation of certain genes with RNA molecules (Link to "riboswitches") , not protein molecules.
| Representative amino acids found in the Murchison meteorite. Six of the amino acids (blue) are found in all living things, but the others (yellow) are not normally found in living matter here on earth. The same amino acids are produced in discharge experiments like Miller's. |
|---|
| Glycine |
Glutamic acid |
| Alanine |
Isovaline |
| Valine |
Norvaline |
| Proline |
N-methylalanine |
| Aspartic acid |
N-ethylglycine |
This meteorite, that fell near Murchison, Australia on 28 September 1969, turned out to contain a variety of organic molecules including:
- purines and pyrimidines
- polyols — compounds with hydroxyl groups on a backbone of 3 to 6 carbons such as glycerol and glyceric acid. Sugars are polyols.
- the amino acids listed here. The amino acids and their relative proportions were quite similar to the products formed in Miller's experiments.
The question is: were these molecules simply terrestrial contaminants that got into the meteorite after it fell to earth.
Probably not:
- Some of the samples were collected on the same day it fell and subsequently handled with great care to avoid contamination.
- The polyols contained the isotopes carbon-13 and hydrogen-2 (deuterium) in greater amounts than found here on earth.
- The samples lacked certain amino acids that are found in all earthly proteins.
- Only L amino acids occur in earthly proteins, but the amino acids in the meteorite contain both D and L forms (although L forms were slightly more prevalent).
This meteorite arrived here from Mars. It contained not only a variety of organic molecules, including polycyclic aromatic hydrocarbons, but — some claim — evidence of microorganisms as well.
Furthermore, there is evidence that its interior never rose about 40° C during its fiery trip through the earth's atmosphere. Live bacteria could easily survive such a trip.
Astronomers, using infrared spectroscopy, have identified a variety of organic molecules in interstellar space, including
- methane (CH4),
- methanol (CH3OH),
- formaldehyde (HCHO),
- cyanoacetylene (HC3N) (which in spark-discharge experiments is a precursor to the pyrimidine cytosine).
- polycyclic aromatic hydrocarbons
- as well as such inorganic building blocks as carbon dioxide (CO2), carbon monoxide (CO), ammonia (NH3), hydrogen sulfide (H2S), and hydrogen cyanide (HCN).
There have been several reports of producing amino acids and other organic molecules by taking a mixture of molecules known to be present in interstellar space such as:
- ammonia (NH3)
- carbon monoxide (CO)
- methanol (CH3OH) and
- water (H2O)
- hydrogen cyanide (HCN)
and exposing it to
- a temperature close to that of space (near absolute zero)
- intense ultraviolet (uv) radiation.
- Whether or not life arrived here from space, there is little doubt that organic matter continuously rains down on the earth (estimated at 30 tons per day).
- Even if life did arrive from space, the fundamental questions remain unanswered: how was it created out there?
31 December 2004
 Stanley Miller, a graduate student in biochemistry, built the apparatus shown here. He filled it with
Stanley Miller, a graduate student in biochemistry, built the apparatus shown here. He filled it with 