| Index to this page |
Here is an overview.
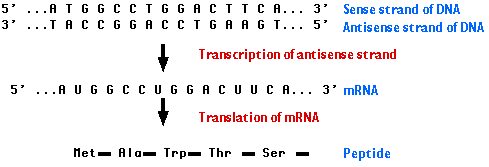
This page examines the first step:
Note that at any place in a DNA molecule, either strand may be serving as the template; that is, some genes "run" one way, some the other (and in a few remarkable cases, the same segment of double helix contains genetic information on both strands!). In all cases, however, RNA polymerase proceeds along a strand in its 3′ → 5′ direction.
| A report in the 4 January 2001 issue of Nature shows that RNA polymerase actually tracks around the double helix of DNA. In vitro, at least, when RNA polymerase is immobilized, it spins the DNA molecule around and around as it moves along the molecule. Whether it is the polymerase or the DNA that does the spinning in vivo remains to be determined. |
The S number given each type of rRNA reflects the rate at which the molecules sediment in the ultracentrifuge. The larger the number, the larger the molecule (but not proportionally).
The 28S, 18S, and 5.8S molecules are produced by the processing of a single primary transcript from a cluster of identical copies of a single gene. The 5S molecules are produced from a different cluster of identical genes.
| Link to a view showing transcription in the rRNA gene cluster. |
| View the structure of a tRNA that brings alanine into the growing polypeptide. |
Messenger RNA comes in a wide range of sizes reflecting the size of the polypeptide it encodes. Most cells produce small amounts of thousands of different mRNA molecules, each to be translated into a peptide needed by the cell.
Many mRNAs are common to most cells, encoding "housekeeping" proteins needed by all cells (e.g. the enzymes of glycolysis). Other mRNAs are specific for only certain types of cells. These encode proteins needed for the function of that particular cell (e.g., the mRNA for hemoglobin in the precursors of red blood cells).
Approximately a dozen different genes for snRNAs, each present in multiple copies, have been identified. The snRNAs have various roles in the processing of the other classes of RNA. For example, several snRNAs are part of the spliceosome that participates in converting pre-mRNA into mRNA by excising the introns and splicing the exons. [Link down to the discussion of RNA processing.]
| Two remarkable reports in the 8 June 2001 issue of Science show the structure of Pol II and reveal many details of how it uses the antisense strand of DNA to synthesize a strand of mRNA. |
All the primary transcripts produced in the nucleus must undergo processing steps to produce functional RNA molecules for export to the cytosol. We shall confine ourselves to a view of the steps as they occur in the processing of pre-mRNA to mRNA.
Most eukaryotic genes are split into segments. In decoding the open reading frame of a gene for a known protein, one usually encounters periodic stretches of DNA calling for amino acids that do not occur in the actual protein product of that gene. Such stretches of DNA, which get transcribed into RNA but not translated into protein, are called introns. Those stretches of DNA that do code for amino acids in the protein are called exons. Examples:
In general, introns tend to be much longer than exons. An average eukaryotic exon is only 140 nts long, but one human intron stretches for 480,000 nucleotides!
Removal of the introns — and splicing the exons together — are among the essential steps in synthesizing mRNA.
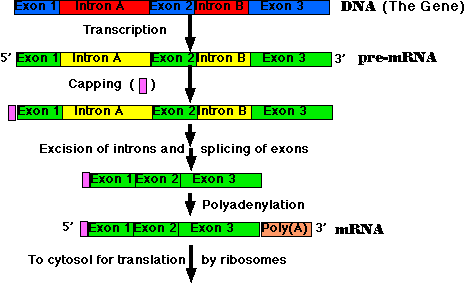
The steps of RNA processing:
The cutting and splicing of mRNA must be done with great precision. If even one nucleotide is left over from an intron or one is removed from an exon, the reading frame from that point on will be shifted, producing new codons specifying a totally different sequence of amino acids from that point to the end of the molecule (which often ends prematurely anyway when the shifted reading frame generates a STOP codon).
The removal of introns and splicing of exons is done with the spliceosome. This is a complex of several snRNA molecules and some 145 different proteins.
The introns in most pre-mRNAs begin with a GU and end with an AG. Presumably these short sequences assist in guiding the spliceosome.
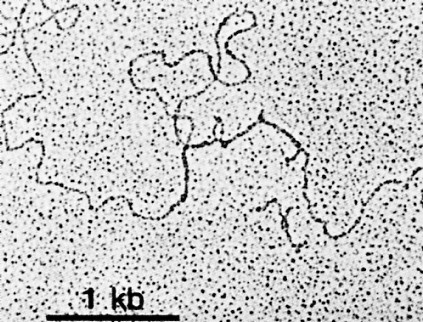
The upper image is an electron micrograph of a mRNA-DNA hybrid molecule formed by mixing the messenger RNA (mRNA) from a clone of antibody-secreting cells with single-stranded DNA from the same kind of cells. The bar represents the length of 1000 bases.
The lower diagram is an interpretation of the micrograph. The solid line represents the DNA; the dotted line the mRNA. The loops (IA, IB, etc.) represent the introns that separate the exons encoding the domains of an antibody heavy chain:The unhybridized portion of the mRNA is its poly(A) tail.
[From R. Maki et al., Proc. Natl. Acad. Sci. USA 77:2138, 1980.]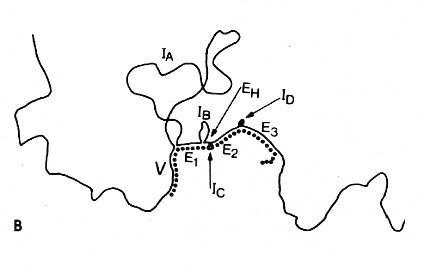
The processing of pre-mRNA for many proteins proceeds along various paths in different cells or under different conditions. For example, early in the differentiation of a B cell (a lymphocyte that synthesizes an antibody) the cell first uses an exon that encodes a transmembrane domain that causes the molecule to be retained at the cell surface. Later, the B cell switches to using a different exon whose domain enables the protein to be secreted from the cell as a circulating antibody molecule.
Alternative splicing provides a mechanism for producing a wide variety of proteins from a small number of genes.
While we humans may turn out to have only 25 to 30 thousand genes, we probably make at least 10 times that number of different proteins. More than 50% of our genes produce pre-mRNAs that are alternatively-spliced.
One of the most dramatic examples of alternative splicing is the Dscam gene in Drosophila. This single gene contains some 116 exons of which 17 are retained in the final mRNA. Some exons are always included; others are selected from an array. Theoretically this system is able to produce 38,016 different proteins. And, in fact, over 18,000 different ones have been found in Drosophila hemolymph.
These Dscam proteins are involved in
Perhaps the incredible diversity of synaptic junctions in the mammalian c.n.s. (~1014) is mediated by alternative splicing of a limited number of gene transcripts.
So, whether a particular segment of RNA will be retained as an exon or excised as an intron can vary under different circumstances. Clearly the switching to an alternate splicing pathway must be closely regulated.
Perhaps during evolution, eukaryotic genes have been assembled from smaller, primitive genes - today's exons. Some proteins, like the antibodies mentioned in the previous section, are organized in a set of separate sections or domains each with a special function to perform in the complete molecule. Each domain is encoded by a separate exon. Having the different functional parts of the antibody molecule encoded by separate exons makes it possible to use these units in different combinations. Thus a set of exons in the genome may be the genetic equivalent of the various modular pieces in a box of "Lego" for children to assemble in whatever forms they wish.
But the boundaries of other exons do not seem to correspond to domain boundaries of the protein. Furthermore, rRNA and tRNA genes are also split, and these do not encode proteins. So perhaps some exons are simply "junk" DNA that was inserted into the gene at some point in evolution without causing any harm.
| Welcome&Next Search |