BIOL 1400 -- Lecture Outline 21
"A person has only so much knowledge as he puts to work." -- St. Francis of Assisi
I. Proteins -- a huge and complicated subject in itself!
- Before I can finish explaining what DNA actually does, we need to look
at the activities of an extremely diverse set of complex molecules called proteins
- Functions of proteins: What don't they do?
- Proteins are important in structure and support
(e.g. the collagen protein in your tendons, the keratin proteins in your skin and
hair).
- Other functions include movement (e.g. the actin and myosin
proteins that make up your muscles). . .
- . . . chemical regulation (e.g. the insulin protein
that regulates blood sugar, unless you're diabetic). . .
- . . . transport (e.g. the hemoglobin
protein that carries oxygen in your bloodstream). . .
- . . . defense (e.g. certain proteins in rattlesnake venom). . .
- One major function of proteins is in driving and controlling other chemical
reactions (such as breaking down foods that you eat, or building new molecules).
Proteins that do this are enzymes. More about enzymes later!
- The monomers that make up proteins are called amino acids.
- Each amino acid has one amino group (NH2-)
and one carboxyl group (-COOH), both attached to a central carbon atom.
- Each amino acid also has another group of atoms, called the R-group,
attached to the central carbon.
- The R-group can be very simple or quite complex. Like so:
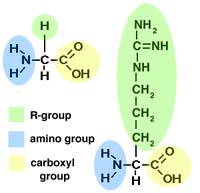
Two sample amino acids (glycine on the left, arginine on the right)
- An amino group on one amino acid may attach to the carboxyl group on another.
This is called a peptide bond -- and another name for a polymer of amino
acids is polypeptides.
- There are twenty amino acids used in almost all proteins. Each has a different
R-group.
- What makes proteins so complex is that a polypeptide may take on an extremely
complex shape.
- The sequence of amino acids is a protein's primary structure. The
picture below, a so-called stick model of a molecule, shows peptide
bonds in blue and carbon atoms in green.
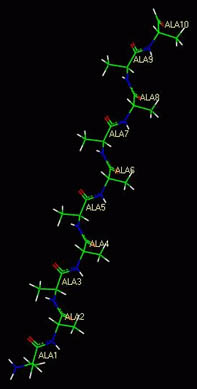
A polymer of animo acids
- Weak attractions between amino acids will cause
an amino acid chain to fold or curl up into its secondary structure. (Examples
of secondary structure are the alpha-helix and
the beta-sheet.)
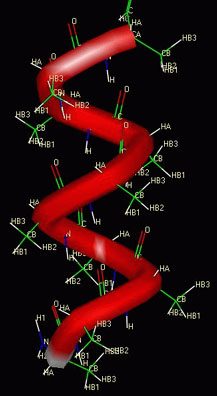
A polymer of animo acids coiled up in an alpha-helix

A polymer of animo acids folded up in a beta-sheet
- R-groups may attract or repel each other, or even form chemical bonds
with each other, which puts kinks or loops in the polypeptide. This causes the
protein to fold up even further. This is its tertiary structure.
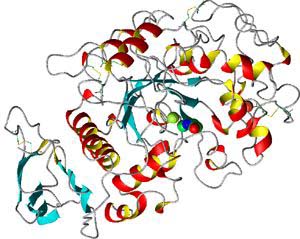
A protein's tertiary structure. You can see several alpha-helix regions
(red and yellow) and beta-sheet regions (blue)
- And finally, different amino acid chains may attach to each other, to form a large
molecule; this is the quaternary structure.
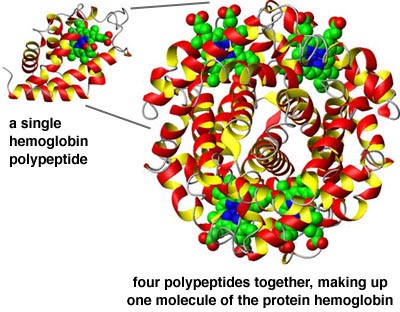
The protein hemoglobin is made up of four chains
II. What's the POINT of all this???
- The POINT is this: The shape of a protein molecule enables it to function.
And the order of amino acids in a protein determines its shape.
- EXAMPLES:
- Molecules of the protein collagen are shaped like long twisted
ropes -- and their function is to connect cells and tissues together. Your tendons,
the non-calcium part of your bones, and the loose tissue under the skin of your
face, are largely collagen. The disease Marfan's syndrome results from
incorrectly shaped collagen molecules.
- Molecules of the protein hemoglobin transport oxygen to all parts
of your body. The disease sickle cell anemia results from incorrectly shaped
hemoglobin proteins. (So do some less well-known diseases.)
- Molecules of the protein hexaminidase break down certain lipids.
The disease Tay-Sachs syndrome results from incorrectly shaped hexaminidase
molecules.
- And so on. . .
- Thus it is extremely important that any living organism be able to
assemble amino acids in the right order -- otherwise it won't be able to
function.
- In the 1900s, Archibald Garrod proposed that each gene, whatever it was, was
responsible for making one protein. A faulty gene would produce a faulty protein.
This is the aptly named one-gene, one-protein hypothesis.
- Garrod and others traced a number of human diseases to faults in a single
protein. For example, the inability to make a single protein called phenylalanine
hydroxylase means that a human can't process the amino acid phenylalanine -- leading
to a toxic buildup in the body called phenylketonuria.
(More information. . .)
- A gene is now known to be a length of DNA.
- Each gene contains the information needed to make one particular
type of protein (whether the protein is an enzyme or something else).
- If you have a faulty gene, that means that the gene will code for a faulty
protein -- one with the wrong order of amino acids, and therefore the wrong shape.
- If you change a protein's shape -- in particular its tertiary structure --
this is called denaturing the protein.
- EXAMPLES: Changing the acidity (e.g. adding vinegar or lemon juice to
milk to make it curdle), heating (e.g. cooking egg white to make it solid) and
certain chemical treatments (e.g. getting a perm) are examples of denaturing
proteins.
III. Enzymes
- Energy takes a lot of forms: heat, light, motion, electricity. . .
- Energy can also be stored in chemical bonds between atoms. That
energy is released when the bonds are broken. On the other hand, energy is also
required to form chemical bonds.
- Many chemical reactions release more energy than they consume. . .
- Example: When you burn gasoline, what you're actually doing is breaking
bonds between the carbon and hydrogen atoms in the gasoline molecules, and
forming new bonds between these atoms and atoms of oxygen in the atmosphere.
Assuming it's a clean burn, the reaction looks something like this:
2 C8H18 + 25 O2 ->
16 CO2 + 18 H2O + heat and light energy
- A reaction like this, once started, will continue spontaneously -- gasoline
will burn until all the gasoline (and/or all the available oxygen) have been
consumed.
- But a reaction may still need an input of energy to get started --
a "kickstart," if you will. This is the activation energy.
- Example: You have to light a match, or fire an electric spark, to start gasoline burning.
- However, some substances will lower the activation energy of a reaction,
making it much easier to start. These are catalysts.
- Try this at home: Try setting a sugar cube on fire with a match. It probably
won't work. Next, rub the sugar cube with a little paper ash, and try lighting it
again. It should work this time. The ash is acting as a catalyst.
- Example 2: The catalytic converter of a modern car engine contains platinum.
Platinum acts as a catalyst to speed up the conversion of harmful carbon monoxide
to relatively harmless carbon dioxide.
- The point of all this is that some of the most important proteins made are
catalysts -- they encourage certain reactions to happen inside a cell.
Proteins that act as catalysts are enzymes.
- An enzyme might bring together two molecules so that they can form a bond
and join together (such as the enzymes that assist in replicating DNA and in
making mRNA). . .
- . . . or it might "stretch" one molecule so that it breaks into two (such as the
enzymes that break down the food that you eat)
- In either case, since the protein is a catalyst, it is not used up by the reaction.
Go to Previous Notes |
Return to Lecture Schedule |
Return to Syllabus |
Contact the Prof |
Go to Next Notes





