Bortezomib (Velcade®)
On 13 May 2003, the U.S. Food and Drug Administration (FDA) approved a drug called bortezomib (Velcade®) (formerly known as LDP-341) to treat patients with multiple myeloma, a cancer of plasma cells.
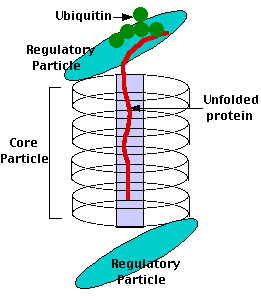
The drug blocks the proteolytic action of the proteasome.
- The failure to degrade IκB blocks the signaling action of the transcription factor NF-κB. [Discussion] Dozens of genes needed for proliferation and adhesion of myeloma cells are turned off.
- The failure to degrade cyclins inhibits completion of the cell cycle and hence the mitotic proliferation of the cancerous cells.
- The drug seems to work especially well when used with conventional "chemo" drugs probably by inhibiting the ability of the cancer cell to protect itself against the damage the "chemo" causes.
- Inhibition of Bcl-2 leads to death of the cell by apoptosis.
- Angiogenesis and metastasis are also inhibited.
The drug is given intermittently, and its action is reversible. Cancer cells are killed while normal cells are spared.
|