Antigens are macromolecules that elicit an immune response in the body. Antigens can be
Most of this page will describe how protein antigens are presented to the immune system.
The presentation of lipid and polysaccharide antigens will be mentioned at the end. [Link]
It will be helpful to distinguish between
- antigens that enter the body from the environment; these would include
- inhaled macromolecules (e.g., proteins on cat hairs that can trigger an attack of asthma in susceptible people)
- ingested macromolecules (e.g., shellfish proteins that trigger an allergic response in susceptible people)
- molecules that are introduced beneath the skin (e.g., on a splinter or in an injected vaccine)
- antigens that are generated within the cells of the body; these would include
- proteins encoded by the genes of viruses that have infected a cell
- aberrant proteins that are encoded by mutant genes; such as mutated genes in cancer cells
In all cases, however, the initial immune response to any antigen absolutely requires that the antigen be recognized by a T lymphocyte ("T cell"). The truth of this rule is clearly demonstrated in AIDS: the infections (viral or fungal or bacterial) that so often claim the life of AIDS patients do so when the patient has lost virtually all of his or her CD4+ T cells.
The two categories of antigens are processed and presented to T cells by quite different mechanisms.
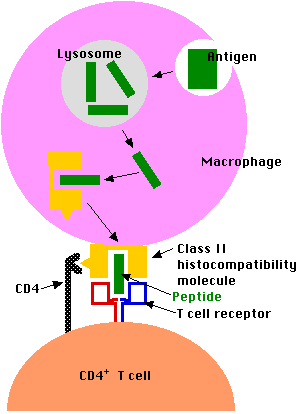
Exogenous antigens (inhaled, ingested, or injected) are taken up by antigen-presenting cells (APCs). These include:
Antigen-presenting cells
- engulf the antigen by endocytosis.
- The endosome fuses with a lysosome where the antigen is
- degraded into fragments (e.g. short peptides).
- These antigenic peptides are then displayed at the surface of the cell nestled within a
- class II histocompatibility molecule.
- Here they may be recognized by CD4+ T cells.
(Dendritic cells can also present intact antigen directly to B cells. In this case, the engulfed antigen is not degraded in lysosomes but is returned to the cell surface for presentation to B cells bearing BCRs of the appropriate specificity.)
Second Group: Endogenous antigens
Antigens that are generated within a cell (e.g., viral proteins in any infected cell) are
- degraded into fragments (e.g., peptides) within the cell and
- displayed at the surface of the cell nestled within a
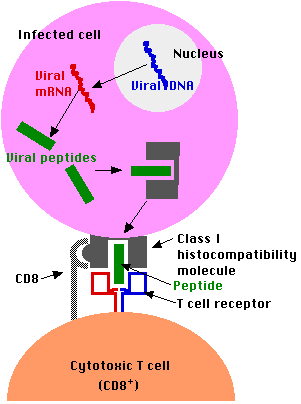
- class I histocompatibility molecule.
- Here they may be recognized by CD8+ T cells.
- Most CD8+ T cells are cytotoxic.
- They have the machinery to destroy the infected cell (often before it is able to release a fresh crop of viruses to spread the infection). [Link to discussion.]
Now for more details.
Class I histocompatibility molecules are transmembrane proteins expressed at the cell surface. Like all transmembrane proteins, they are synthesized by ribosomes on the rough endoplasmic reticulum (RER) and assembled within its lumen.
There are three subunits in each class I histocompatibility molecule:
- the transmembrane polypeptide (called the "heavy chain")
- the antigenic peptide
- beta-2 microglobulin
All of these must be present within the lumen of the endoplasmic reticulum if they are to assemble correctly and move through the Golgi apparatus to the cell surface.
The Problem: proteins encoded by the genes of an infecting virus are synthesized in the cytosol. How to get them into the endoplasmic reticulum?
The Solution: TAP (= transporter associated with antigen processing).
- Viral proteins in the cytosol are degraded by proteasomes into viral peptides.
- The peptides are picked up by TAP proteins embedded in the membrane of the endoplasmic reticulum.
| TAP proteins are members of the ABC group of membrane transporters. Discussion. |
- Using the energy of ATP, the peptides are pumped into the lumen of the endoplasmic reticulum where they assemble with
- the transmembrane polypeptide and beta-2 microglobulin.
- This trimolecular complex then moves through the Golgi apparatus and is inserted in the plasma membrane.
- The complex can be bound by a T cell with
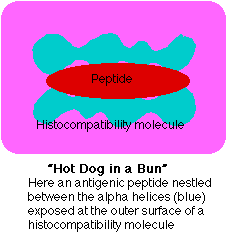
- a receptor (TCR) able to bind the peptide and flanking portions of the histocompatibility molecule (the hot dog in the bun) and
- CD8 molecules that bind the CD8 receptor (shown above as a gray hemisphere) on the histocompatibility molecule.
Class II histocompatibility molecules consist of
- two transmembrane polypeptides and
- a third molecule nestled in the groove they form.
All three components of this complex must be present in the endoplasmic reticulum for proper assembly.
But antigenic peptides are not transported to the endoplasmic reticulum, so a protein called the invariant chain ("Ii") temporarily occupies the groove.
The steps:
- The two chains of the class II molecule are inserted into the membrane of the endoplasmic reticulum.
- They bind (in their groove) one molecule of invariant chain.
- This trimolecular complex is transported through the Golgi apparatus and into vesicles called lysosomes.
Meanwhile,
- Foreign antigenic material is engulfed by endocytosis forming endosomes.
- These also fuse with lysosomes.
Then,
- The antigen is digested into fragments.
- The invariant (Ii) chain is digested.
- This frees the groove for occupancy by the antigenic fragment.
- The vesicles move to the plasma membrane and the complex is displayed at the cell surface.
- The complex can be bound by a T cell with
- a receptor (TCR) able to bind the peptide and flanking portions of the histocompatibility molecule (the hot dog in the bun) and
- CD4 molecules that bind the CD4 receptor (shown above as a yellow triangle) found on all class II histocompatibility molecules.
|
External Link |
To see other animations of these processes, click on immunobiology
and navigate to "Antigen Recognition" → "MHC class I processing"
and "MHC class II processing" respectively. (Requires Flash 6). |
| Please let me know by e-mail if you find a broken link in my pages.) |
Transferring viral antigens to Antigen-Presenting Cells (APCs)
"Professional" antigen-presenting cells (APCs) like dendritic cells can use the class I as well as the class II pathways of antigen presentation.
This is fortunate because:
- Most viruses infect cells other than APCs.
- While viral antigens displayed on the surface of infected cells can serve as targets for cytotoxic T cells (CTLs),
- the lack of any costimulatory molecules on the cell surface makes them poor stimulants for the development of clones of CTLs in the first place.
However, at least two mechanisms exist for transferring viral antigens from any infected cell to a professional APC.
- When an infected cell dies, it can be engulfed by a professional APC and the viral antigens within it can enter the class I pathway.
- The dead cell is engulfed by phagocytosis as described above.
- The endosome that forms fuses with a lysosome and degradation of the dead cell begins.
- Viral antigens pass into the cytosol and are degraded in proteasomes.
- The viral peptides formed are then are picked up by TAP and, as described above,
- inserted into class I MHC molecules and
- displayed at the cell surface — along with the costimulatory molecules needed to start a vigorous clonal expansion of CD8+ cytotoxic T cells.
- Cells infected with viruses can also transfer viral peptides directly from their cytosol to an adjacent cell like
- a professional APC able to present the peptide — with the needed costimulatory molecules — to CTLs;
- or simply a cell of the same type that can then present it in a class I molecule and be killed by a CTL before the infection can spread to it. This mechanism provides a way of walling off the infection.
- In both cases, the transfer occurs through gap junctions linking the adjacent cells.
Autophagy [Link] provides a mechanism by which cells can transfer intracellular antigens (e.g., proteins synthesized by an infecting virus) into the class II pathway in addition to class I. In this way viral infection can generate CD4+ T cells as well as cytotoxic T cells (CD8+).
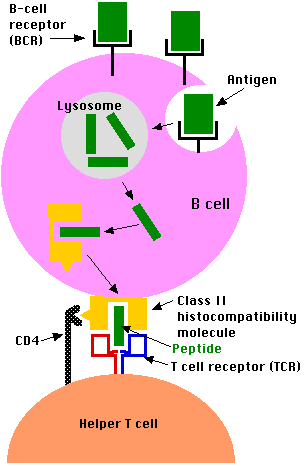
B lymphocytes process antigen by the class II pathway.
However, antigen processing by B cells differs from that of phagocytic cells like macrophages in crucial ways:
- B cells engulf antigen by receptor-mediated endocytosis
- The B cell receptors for antigen (BCRs) are antibodies anchored in the plasma membrane.
- The affinity of these for an epitope on an antigen may be so high that the B cell can bind and internalize the antigen when it is present in body fluids in concentrations thousands of times smaller than a macrophage would need.
- The remaining steps of antigen processing occur by the same class II pathway described above for macrophages producing
- fragments of antigen displayed at the cell surface nestled in the groove of class II histocompatibility molecules.
- A CD4+ T cell that recognizes the displayed antigen is stimulated to release lymphokines.
- These, in turn, stimulate the B cell to enter the cell cycle.
- Because of the part they play in stimulating B cells, these CD4+ T cells are called Helper T cells ("Th").
- The B cell grows into a clone of cells (called plasma cells)
- These synthesize receptors (BCRs) with the identical binding site for the epitope but without the transmembrane tail.
- These antibodies are secreted into the surroundings.
Lipid Antigens
- Lipid antigens are presented to T cells by cell-surface molecules designated CD1 ("cluster of differentiation" 1).
- Antigen-presenting cells express several different forms of CD1 at their surface. Each is probably specialized to bind a particular type of lipid antigen (e.g. lipopeptide vs glycolipid).
- The exposed surface of CD1 molecules forms an antigen-binding groove much like that of MHC molecules except that
- the amino acids in the groove are more hydrophobic than those in MHC molecules.
- Like protein antigens, lipid antigens are also presented as fragments, i.e., as a "hot dog in a bun".
Polysaccharide Antigens
Some bacterial polysaccharides ingested by APCs
- can be degraded in their lysosomes
- and presented to T cells by MHC class II molecules.
Nitric oxide (NO) appears to be essential for this process.
4 December 2005



