| Index to this page |
The period between M and S is called G1; that between S and M is G2.
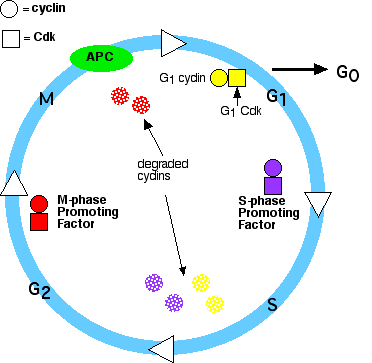 So, the cell cycle consists of:
So, the cell cycle consists of:
Their levels in the cell rise and fall with the stages of the cell cycle.
Their levels in the cell remain fairly stable, but each must bind the appropriate cyclin (whose levels fluctuate) in order to be activated.
They add phosphate groups to a variety of protein substrates that control processes in the cell cycle.
| Link to discussion of proteasomes. |
| This is only one mechanism by which the cell ensures that every portion of its genome is copied once — and only once — during S phase. Link to discussion. |
| Some cells deliberately cut the cell cycle short allowing repeated S phases without completing mitosis and/or cytokinesis. This is called endoreplication and is described on a separate page. Link to it. |
| Discussion of DNA replication. |
| Link to discussion of the roles of the spindle in mitosis. |
| Link to description of the events in mitosis. |
All the checkpoints examined require the services of a complex of proteins. Mutations in the genes encoding some of these have been associated with cancer; that is, they are oncogenes. This should not be surprising since checkpoint failures allow the cell to continue dividing despite damage to its integrity.
| Further discussion of tumor suppressor genes and p53. |
The p53 protein is also a key player in apoptosis, forcing "bad" cells to commit suicide. So if the cell has only mutant versions of the protein, it can live on — perhaps developing into a cancer. More than half of all human cancers do, in fact, harbor p53 mutations and have no functioning p53 protein.
| A genetically-engineered adenovirus, called ONYX-015, can only replicate in human cells lacking p53. Thus it infects, replicates, and ultimately kills many types of cancer cells in vitro. Clinical trials are now proceeding to see if injections of ONYX-015 can shrink a variety of types of cancers in human patients. |
In some way, p53 seems to evaluate the extent of damage to DNA, at least for damage by radiation.
ATM (="ataxia telangiectasia mutated") gets its name from a human disease of that name [Link], whose patients — among other things — are at increased risk of cancer. The ATM protein is involved in
MAD (="mitotic arrest deficient") genes (there are two) encode proteins that bind to each kinetochore until a spindle fiber (one microtubule will do) attaches to it. If there is any failure to attach, MAD remains and blocks entry into anaphase.
| Link to discussion of chromosome behavior in anaphase. |
Mutations in MAD produce a defective protein and failure of the checkpoint. The cell finishes mitosis but produces daughter cells with too many or too few chromosomes (aneuploidy). Aneuploidy is one of the hallmarks of cancer cells suggesting that failure of the spindle checkpoint is a major step in the conversion of a normal cell into a cancerous one.
Infection with the human T cell leukemia virus-1 (HTLV-1) leads to a cancer (ATL = "adult T cell leukemia") in about 5% of its victims. HTLV-1 encodes a protein, called Tax, that binds to MAD protein causing failure of the spindle checkpoint. The leukemic cells in these patients show many chromosome abnormalities including aneuploidy.
A kinesin that moves the kinetochore to the end of the spindle fiber also seems to be involved in the spindle checkpoint [More].
Many times a cell will leave the cell cycle, temporarily or permanently. It exits the cycle at G1 and enters a stage designated G0 (G zero). A G0 cell is often called "quiescent", but that is probably more a reflection of the interests of the scientists studying the cell cycle than the cell itself. Many G0 cells are anything but quiescent. They are busy carrying out their functions in the organism. e.g., secretion, attacking pathogens.
Often G0 cells are terminally differentiated: they will never reenter the cell cycle but instead will carry out their function in the organism until they die.
For other cells, G0 can be followed by reentry into the cell cycle. Most of the lymphocytes in human blood are in G0. However, with proper stimulation, such as encountering the appropriate antigen, they can be stimulated to reenter the cell cycle (at G1) and proceed on to new rounds of alternating S phases and mitosis.
G0 represents not simply the absence of signals for mitosis but an active repression of the genes needed for mitosis. Cancer cells cannot enter G0 and are destined to repeat the cell cycle indefinitely. [More]
| Welcome&Next Search |