| Index to this page |
|
| Loss of structure and function in aging. Figures represent percentage of a given function remaining in an average 75-year-old man compared with that found in an average 30-year-old man, the latter value taken as 100%. | |
|---|---|
| Weight of brain | 56% |
| Blood supply to brain | 80 |
| Output of heart at rest | 70 |
| Number of glomeruli in kidney | 56 |
| Glomerular filtration rate | 69 |
| Speed of return to normal pH of blood after displacement | 17 |
| Number of taste buds | 36 |
| Vital capacity | 56 |
| Strength of hand grip | 55 |
| Maximum O2 uptake during exercise | 40 |
| Number of axons in spinal nerve | 63 |
| Velocity of nerve impulse | 90 |
| Body weight | 88 |
This table gives some data.
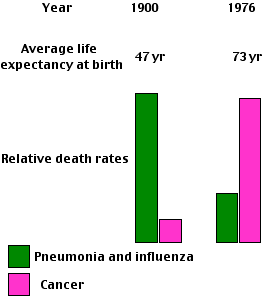
The decline in function certainly occurs within cells. This is especially true of cells that are no longer in the cell cycle:| At the start of the 20th century, infectious diseases such as pneumonia and influenza caused more deaths in the United States than "organic" diseases like cancer. Now the situation is reversed. The availability of effective weapons against infectious disease, e.g., antibiotics and immunization, has greatly increased the average life span (but not maximum life span) and resulted in "organic" diseases like cardiovascular disease and cancer becoming the most common cause of death. |
|---|
 |
In fact, only humans and the animals they choose to protect (pet dogs, cats, parrots, etc.) age.
And even for humans, aging has only become common in recent decades. In 1900, a newborn child in the U.S. could look forward to an average life expectancy of only 47 years. Infectious diseases were the major causes of death, killing most people before they reached an age when aging set in.Three-quarters of a century later, life expectancy had risen to 73 years and "organic" diseases, including all the diseases of aging, had replaced infectious diseases as the major cause of death. Today, the life expectancy has risen to 80 for women (74 for men), and coping with an aging population has become a major economic and social challenge in the U.S. [Link]
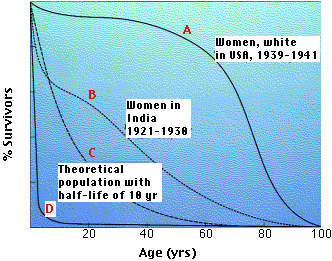
Organisms with survivorship curves between C and D have no opportunity to show the signs of aging.
In every case, these animals have no fixed size but continue to grow throughout life. This, of course, requires a constant supply of new cells, and this may be their secret.
The situation is different for the warm-blooded vertebrates, the birds and mammals.They do have a fixed adult size and, if protected from environmental hazards, will show signs of aging.
Genes that suppress signaling by insulin and insulin-like growth factor-1 (Igf-1) increase life span in these animals.
Examples:Why should this be? Three (interrelated) possibilities:
These contradictory results do not negate the role of genes in aging, but indicate that other environmental factors (e.g. more food left for the survivors) may skew the outcome.
The life span of yeast, C. elegans, Drosophila, birds, and mammals (mice, rats, and probably monkeys) can be extended, and signs of aging delayed, if they are maintained on a semi-starvation diet.
The increased life span of yeast subjected to calorie restriction requires a gene called SIR2 ("Silent Information Regulator 2"). SIR2 encodes the Sir2 deacetylase, an enzyme that removes acetyl groups from proteins. Increasing the activity of Sir2 extends the life span of yeast, C. elegans, and Drosophila.It might seem unlikely that we could learn anything about longevity in mammals from studying yeast. But it turns out that mammals have a gene similar to SIR2 (called Sirt1 in mice).
Resveratrol, a small molecule found in red wine, activates the Sir2 enzyme and extends life span in yeast. It may do the same in Drosophila and C. elegans. In human cells, resveratrol activates Sirt1 mimicking the effects of calorie restriction. But before rushing out to buy red wine, realize that, at least in mice, the Sirt1 protein inhibits the production of the tumor-suppressor protein p53. Mice expressing high levels Sirt1 are more susceptible to cancer.
Calorie restriction in mice causes| Link to discussion of reactive oxygen species. |
| Link to a discussion of the effect of ROS on lipids |
But it may be damage to DNA that is the crucial factor in the decline in cell function with age. [Link]
In any case, transgenic mice containing the human gene for catalase (but with the targeting signal that would normally send the protein to peroxisomes replaced with that for mitochondria) live 20% longer than normal for their strain. [See Schriner, S. E. et al., Science, 24 June 2005]
One might expect that cells removed from a mouse or human and placed in tissue culture could be cultured indefinitely just as bacteria can. But that is not the case.
When human fibroblasts, for example, are placed in culture, they proliferate at first, but eventually a time comes when their rate of mitosis slows and finally stops. The cells continue to live for a while, but cannot pass from G1 to the S phase of the cell cycle. This phenomenon is called replicative senescence. Fibroblasts taken from a young human pass through some 60–80 doublings before they reach replicative senescence.
Why should this be?
Cells — unless they retain the enzyme telomerase — lose DNA from the tips of their chromosomes (telomeres) with each cell division. In general, the telomeres in the cells of old animals are much shorter than those in young animals.
A recent study of short-lived versus long-lived birds showed that telomere shortening was faster in the short-lived species. And one species, a petrel which lives four times as long as other birds of its size, actually has telomeres that grew longer with age.
Most somatic cells of the body cease to express telomerase. (Germ cells, some stem cells, and cancer cells continue to express the enzyme.)
If telomeres get too short, chromosome abnormalities — a hallmark of cancer — occur. These can be avoided if the cell senses this dangerous condition and ceases to divide. The p53 protein may mediate the signal calling for stopping the cell cycle. The result: replicative senescence.
Evidence: Cells genetically manipulated to express telomerase long after they should have stopped, avoid replicative senescence.
So replicative senescence may be the price we pay for removing cells from the cell cycle before they can accumulate the mutations that would turn them into cancer cells.
The role of the p53 protein in replicative senescence is mirrored in the intact animal, at least in mice. Mice that express abnormally-high levels of p53 activity show many signs of premature aging.
How would replicative senescence of cells lead to the deterioration in structure and function of the aging tissues (e.g., skin) in which they reside? In tissues, e.g., skin and other epithelia, where mitosis must continue throughout life to replace the cells that are lost, the accumulation of senescent cells —incapable of further mitosis — could leading to the characteristic changes of aging in that tissue.
| Effect of radiation on aging. These mice are all 14 months old. As young adults, nine mice were given sublethal doses of radiation and nine others were left as untreated controls. The control mice (left) are still sleek and vigorous at 14 months, while six of the irradiated mice have died and the remaining three show signs of extreme aging (right). [Research photographs of Dr. Howard J. Curtis.] | |
|---|---|
 |
 |
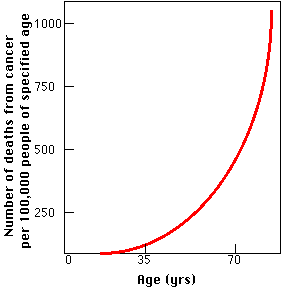
A group of Harvard researchers reported (in the 26 June 2004 issue of Nature) the results of their study of gene expression in the human brain.
They extracted the RNA from autopsied brain tissue of 30 people who had died at ages ranging from 26 to 106. They analyzed the RNA with DNA chips looking for the level of activity of some 11,000 different genes (the transcriptome). A clear pattern emerged.
The level of activity of some 400 genes changed over time.
Humans suffer from a number of rare genetic diseases that, among other things, produce signs of premature aging, e.g., gray hair, wrinkled skin, and shortened life span. In several cases, the mutated genes are ones that have roles to play in maintaining the integrity of the genome, that is, in DNA repair.
Hutchinson-Gilford progeria syndrome does not seem to involve DNA repair. However, nuclear lamins, do anchor chromosomes and perhaps defective lamins can also lead to genome instability.
| Correlation between life span and the relative effectiveness of DNA repair in cells of certain mammals. In each case, cells growing in tissue culture were irradiated with ultraviolet light and then the efficiency with which they repaired their DNA was determined. (From the work of R. W. Hart and R. B. Setlow, 1974.) | ||
|---|---|---|
| Species | Average life span, yr | Relative effectiveness of DNA repair |
| Human | 70 | 50 |
| Elephant | 60 | 47 |
| Cow | 30 | 43 |
| Hamster | 4 | 26 |
| Rat | 3 | 13 |
| Mouse | 2 | 9 |
| Shrew | 1 | 8 |
If aging represents the inevitable consequence of a failure of DNA repair, why does it occur so much sooner in some mammals (e.g., mice) than in others (e.g., elephants and humans)?
The answer probably lies in the risk of death from external factors (e.g., predation, starvation, cold) in that species.
As noted above, few small mammals ever age because they die early of external causes. These animals are r-strategists, putting their energy into quickly| Link to a discussion of r-strategists and K-strategists. |
The table shows that the efficiency of DNA repair is directly correlated with life span in a variety of mammals.
Examining the various factors that have been implicated in the aging process suggests that most —perhaps all — are interrelated.

This pattern in clearly programmed in the genes. Even with plentiful moisture, soil minerals, sunlight, and warm temperatures, the plants age and die.
The situation is quite different in perennials. Throughout their lives, woody perennials (trees) produce new vascular tissue, leaves, and flowers each year. They do not show marked signs of aging, although their rate of growth may decline over the years. Finally, disease or inability to support their ever-increasing size against wind or snow load lead to their death.
This picture (courtesy of Walter Gierasch) is of bristlecone pines (Pinus aristata) growing in the White Mountains of eastern California. Tree-ring analysis shows that many of these trees are over 4000 years old. But note that no living cells in the tree are more than a few years old.
| Welcome&Next Search |