Reaching the goal of effective gene therapies for human diseases has been a difficult one.
Some of the problems that remain to be solved include:
- how to avoid an immune response in the patient, which can interfere with gene therapy in two ways:
- the vector provokes inflammation (a problem with adenovirus vectors)
- the vector elicits antibodies that destroy the vector when it is administered again
- how to get the gene into non-dividing cells like liver, muscle, and neurons;
- how to get the gene integrated into the DNA of the host cell so that it will be replicated (in dividing cells) and expressed indefinitely but
- minimize the risk that it inserts near a proto-oncogene which it could activate producing a cancer. (This occurred in three little boys treated with a retroviral vector based on the murine leukemia virus. [Link])
- how to get the gene to be expressed as needed; that is, how to bring the gene under normal physiological controls so that its product is produced when and in the amounts needed.
Adeno-associated virus gets its name because it is often found in cells that are simultaneously infected with adenovirus. However, by itself it seems to be harmless.
Unlike adenovirus, AAV
- does not stimulate inflammation in the host
- does not elicit antibodies against itself
- can enter non-dividing cells
- integrates successfully into one spot in the genome of its host (on chromosome 19 in humans).
As for the last criterion — how to get the transgene to be expressed appropriately — that may be solved by using two AAV vectors simultaneously:
In the 1 January 1999 issue of Science, James M. Wilson and his colleagues reported the results of using this strategy in both mice and rhesus monkeys.
They injected their experimental animals with two vectors.
This piece of DNA contained (among other things):
- the DNA of adeno-associated virus (AAV)
- a gene encoding a protein containing two domains:
- a portion of the molecule ("p65") that is needed to activate gene transcription but that by itself cannot bind to DNA
- a portion ("FRB") that binds the drug rapamycin.
- a gene encoding another protein with two domains:
- a portion of molecule ("ZFHD1") that binds specifically to the DNA sequence in the promoter of the erythropoietin gene but that by itself cannot activate transcription of the gene;
- a portion ("FKBP12") that also binds to rapamycin.
- promoters (not shown) that allow continuous expression (transcription and translation) of the two genes. But note that, by themselves, the two gene products are inactive.
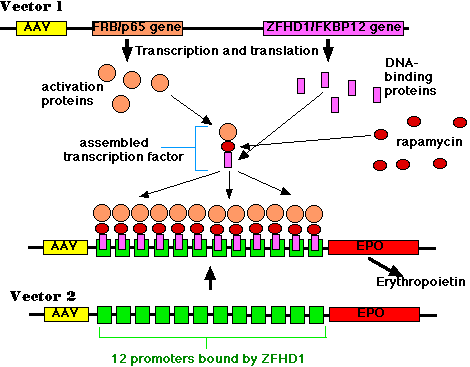
This piece of DNA contained (among other things):
- the DNA of adeno-associated virus (AAV);
- 12 identical promoters (green boxes) of the erythropoietin gene;
- the structural gene for erythropoietin (EPO) itself.
The experimental animals were injected (in their skeletal muscles) with many copies of both vectors. Skeletal muscle was chosen because muscle fibers are multinucleate. Once across the plasma membrane, there are many nuclei which the vectors can enter and hence many opportunities to integrate into the DNA of the host.
Later the animals were injected with rapamycin. This small molecule is an immunosuppressant and is currently being tested in transplant recipients to help them avoid rejection of the transplant. It was used here because of its ability to simultaneously bind to the FRB and FKBP12 domains of the two gene products of vector 1. The resulting trimer is an active transcription factor for the erythropoietin gene.
- injections of the two vectors had — by themselves — no effect on the production of EPO nor on the number of red blood cells (hematocrit), but
- every time these animals were given an injection of rapamycin, they
- quickly began to produce EPO (with levels increasing as much as 100 fold) and
- the number of red blood cells rose (hematocrits increasing from 42% to 60%).
- The amount of EPO produced was directly related to the amount of rapamycin given.
- Even after 5 months, a single injection of rapamycin produced a sharp rise in the level of EPO in the blood.
The results were similar to those in mice, but the effect wore off after 4 months.
So here is a system where a gene introduced into an animal can then be
- switched on by giving the animal a small molecule. (In humans, rapamycin can be given by mouth as a pill.)
- can have its output regulated by the amount of the small molecule administered.
Researchers in Seoul, Korea reported in the 23 November 2000 issue of Nature that they have used an AAV-type vector to cure
- mice with inherited IDDM
- rats with IDDM induced by chemical destruction of their insulin-secreting beta cells.
Both groups of animals were injected (in their hepatic portal vein) with billions of copies of a complex vector containing:
- AAV
- the complementary DNA (cDNA) encoding a synthetic version of insulin
- a promoter that is active only in liver cells and is turned on by the presence of glucose
- the DNA encoding a signal sequence (so that the insulin can be secreted)
- an enhancer to elevate expression of this artificial gene.
The results:
Both groups of animals gained control over their blood sugar level and kept this control for over 8 months. When given glucose, they proceeded to synthesize the synthetic insulin which then brought their blood glucose back down to normal levels.
Researchers at the Salk Institute reported (in the 30 March 1999 issue of the Proceedings of the National Academy of Sciences) work with mice
- whose genes for clotting factor IX had been "knocked out" and
- thus were subject to uncontrolled bleeding like human patients with hemophilia B.
These mice were injected (also in the hepatic portal vein) with DNA containing
- AAV
- cDNA for factor IX (the dog gene)
- liver-specific promoter and enhancer sequences
The mice proceeded to make factor IX and were no longer susceptible to uncontrolled bleeding.
More recently (2005), injection of embryonic stem cells with functioning factor IX genes into the liver of mice without the genes cured them.
ALS (amyotrophic lateral sclerosis) is a human disease in which motor neurons degenerate. (It is often called "Lou Gehrig's disease" after the baseball player who died from it. It is also the disease that Stephen Hawking suffers from.)
A similar disease can be created in transgenic mice carrying mutant human genes (for superoxide dismutase) associated with ALS.
Researchers at the Salk Institute have slowed up the progression of the disease in these mice by injecting their skeletal muscles with an AAV vector containing the gene for insulin-like growth factor 1 (IGF-1).
The vector
The results: destruction of motor neurons was reduced, and the mice lived longer than they otherwise would have.
It's a big jump from mice to humans, but these results indicate that the principle of gene therapy for single-gene disorders is valid.
And some early trials in humans look promising.
- Injections of an AAV vector containing the factor IX gene have temporarily produced functional levels of factor IX in patients with hemophilia B. However, the side effects in a few patients caused the trials to be suspended in May 2004.
| But a word of caution: in one set of earlier experiments, some mice injected with an AAV vector later developed liver tumors. The preliminary evidence is that the injections were not the cause, and human trials with AAV vectors continue. In fact, on August 18, 2003, physicians in New York injected 3.5 x 109 copies of an AAV vector carrying a gene for the synthesis of GABA into the brain of a patient with Parkinson's disease. He is the first of a phase I clinical trial of this procedure. |
- Several children suffering from X-linked severe combined immunodeficiency have had their immune systems restored after retroviral gene therapy. [Link to discussion]
- A few patients with hemophilia A have shown modest improvement when injected with their own cells that had earlier been harvested and transformed in vitro with a plasmid containing the factor VIII gene.
| Link to discussions of other approaches to gene therapy using
|
16 June 2005
