All eukaryotes and some prokaryotes (cyanobacteria) display changes in gene activity, biochemistry, physiology, and behavior that wax and wane through the cycle of days and nights.
Examples:
- the level of the hormone melatonin that rises in your body during the night and falls during the day.
- fruit flies (Drosophila melanogaster) hatch in greatest numbers just at dawn.
Even when the organism is placed in constant conditions (e.g., continuous darkness), these rhythms persist. However, without environmental cues, they tend to be somewhat longer or somewhat shorter than 24 hours — giving rise to the name circadian rhythms (L. circa = about; dies = day).
The genetics and molecular biology of circadian rhythms have been studied in several model organisms including
- some unicellular eukaryotes
- fungi
- plants (Arabidopsis)
- invertebrates (Drosophila)
- mammals (mice, rats, and humans)
What has emerged are some remarkable similarities in mechanisms across these various groups. Let us take a detailed look at the mechanism in Drosophila.
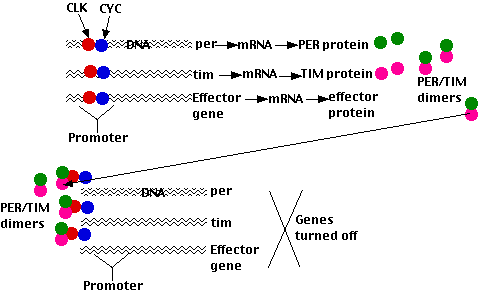 A number of genes in Drosophila are turned on when the animal is exposed to light:
A number of genes in Drosophila are turned on when the animal is exposed to light:
- effector genes whose products mediate the animal's responses (e.g. hatching or molting)
- clock genes whose products regulate the circadian clock. Two key members of this group are:
- period (per)
- timeless (tim)
Activation of all of these genes requires that their promoters are bound by the protein transcription factors
- CLOCK encoded by the gene clock (clk) and
- CYCLE encoded by the gene cycle (cyc)
(The names of proteins will be designated with capitalized Roman letters; the genes that encode them indicated in lower case italics.)
- The PER and TIM proteins (synthesized on ribosomes in the cytoplasm) form dimers.
- When the concentration of these gets high enough (early evening), they are transported into the nucleus.
- Here PER
- binds to the CLK/CYC transcription factors, removing them from the promoters of the genes they activate; thus shutting off transcription.
Because these genes include per and tim, the result is a negative feedback look; that is, the products of the per and tim genes inhibit their own synthesis. Just as the heat of a furnace turns — through the thermostat — its own production off, so a rising level of PER/TIM dimers turns off further synthesis of them. As the level then falls, this inhibition is lifted and PER/TIM activity begins anew.
- turn on clock gene expression.
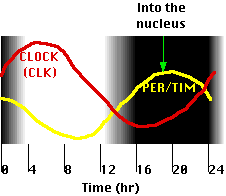
- The time required for the different effects results in the levels of PER/TIM and CLOCK oscillating in opposite phases with a circadian (~24 hr) rhythm (figure).
Even without any external cues (e.g., alternating light and dark), the cycles persist although they tend to drift away from environmental time.
- Under natural conditions, the clocks are precise.
- This is because they are "set" (synchronized) by environmental cues, of which light is one of the most important.
In Drosophila, it works like this.
- Light (blue) is absorbed by the protein cryptochrome (CRY).
- This causes an allosteric change in its conformation enabling it to
- bind to TIM.
- This causes TIM to break down (in proteasomes) ending its inhibition on gene transcription.
- If this happens when PER/TIM levels are rising (late in the "day"), it sets the clock back.
- If it happens when PER/TIM levels are declining (late in the "night"). it sets the clock ahead.
The circadian clock in mammals resembles that in Drosophila in a number of ways with many of the participating genes being homologous. However, there are some differences:
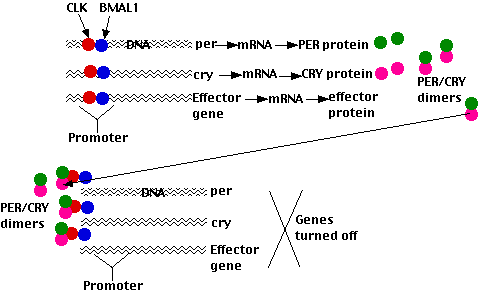
- The transcription factors that turn on the light-induced promoters are dimers of the CLOCK protein and a protein designated BMAL1. These dimers turn on
- the Per gene;
- Cry, the gene encoding cryptochrome
- a gene whose product inhibits transcription of Bmal1
- effector genes (such as the gene encoding arginine vasopressin)
- The Per and Cry mRNAs are exported to the cytoplasm where they are translated.
- The PER and CRY proteins then form dimers that enter the nucleus where they
- turn OFF their own genes (as PER/TIM dimers do in Drosophila);
- turn OFF the gene inhibiting Bmal1 so this double-negative effect causes the level of the BMAL1 protein to rise.
These actions cause the levels of BMAL1 and PER/CRY to oscillate in opposite phases (as CLOCK and PER/TIM do in Drosophila — see the figure above).
Many tissues in mammals, e.g., liver and skeletal muscle, have endogenous clocks. But all of these are under the control (more or less, see note) of a "master clock", the suprachiasmatic nucleus (SCN) — clusters of neurons in the hypothalamus. Small wonder, then, that the blood levels of hormones
- synthesized in the hypothalamus, e.g. arginine vasopressin (also called the antidiuretic hormone, ADH) or
- whose secretion is controlled by the hypothalamus such as
have strong circadian rhythms.
Mice who are totally blind (lacking both rods and cones) have no trouble keeping their circadian clock on time.
They are able to do this because
- some 1–2% of the ganglion cells in their retina — instead of depending on signals arriving from rods and/or cones —
- detect light directly.
- These ganglion cells have an extensive network of dendrites that contain the pigment melanopsin.
- When exposed to light (diffuse light is fine), these ganglion cells become depolarized and send their signals back to the suprachiasmatic nucleus (SCN).
| Note. A group of researchers report in the 19 January 2001 issue of Science that they succeeded in shifting the liver clock of rats using a restricted schedule of feeding (one period per day) while the animals's SCN clock continued to keep time to the cycle of light and dark. The liver is, of course, a major player in the processing of nutrients. [Link] |
Unlike mice, people who are totally blind cannot set the clock in their SCN. As a result, their circadian rhythm drifts out of phase with the actual cycle of day and night. These people often are bothered by feeling sleepy during the day and wide awake when they want to be asleep at night. A recent (12 October 2000) report in the New England Journal of Medicine tells of a group of blind people who were able to set their clocks with the help of a dose (10 mg) of melatonin at bedtime. However, this treatment worked only when the subject's circadian rhythm had drifted so that the normal rise in melatonin from the pineal gland was occurring in the early evening; that is, the dose of melatonin had to be given when it could boost the endogenous level of the hormone.
Some people suffer from a disorder called familial advanced sleep-phase syndrome (FASPS). As the name suggests,
- it is inherited ("familial") and
- their circadian clocks run fast ("advanced").
Those afflicted tend to wake up several (up to four) hours earlier than normal.
One cause of the disorder turns out to be a point mutation in the human PER2 gene. As a result, the PER2 protein builds up more rapidly than normal triggering earlier feedback inhibition.
You can read more about this research at:
And for further details on circadian rhythms and clocks in Drosophila, mammals, and Neurospora, link to
23 February 2005


